Essentially composed of keratin, hair also contains other elements and molecules that contribute to its appearance and behaviour
The overall chemical composition of hair is 45 % carbon, 28 % oxygen, 15 % nitrogen, 7 % hydrogen and 5 % sulphur.
The hair shaft is essentially composed of keratin. Hair keratin is hard, compact and strong. This fibrous protein is gradually formed inside cells from the germinal layer. These cuticle cells are characterised by the presence of amorphous keratin while the cortical cells have a structure of filaments surrounded by a keratinic substance that is richer in sulphur and contains amino acids. The keratin of these filaments forms a helix, with a distance between the turns of 0.51 nanometres and a structure maintained by hydrogen bonds. This protein plays a key role in the cohesion and physical properties of hair.
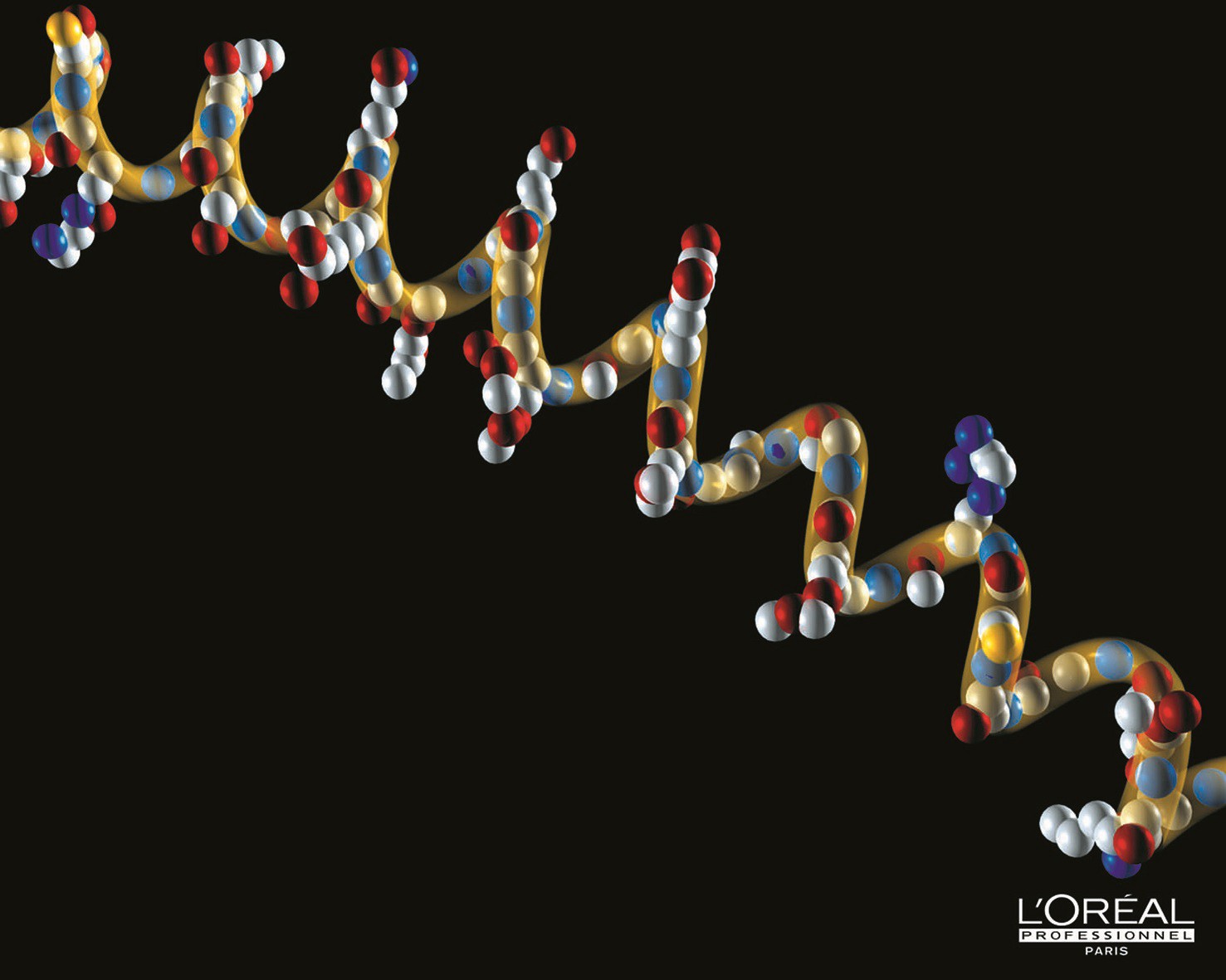 Hair is essentially composed of keratin, a molecule with a helical structure. L?Oral Research
Hair is essentially composed of keratin, a molecule with a helical structure. L?Oral Research
Hair also contains water (12 to 15 %) and traces of mineral elements (calcium, cadmium, chromium, copper, zinc, iron and silicon). These elements can be brought to the base of the hair follicle by blood circulation and then contribute to building the hair shaft. Besides this contribution, the environment can, for example through pollution, be the source of certain elements such as lead.
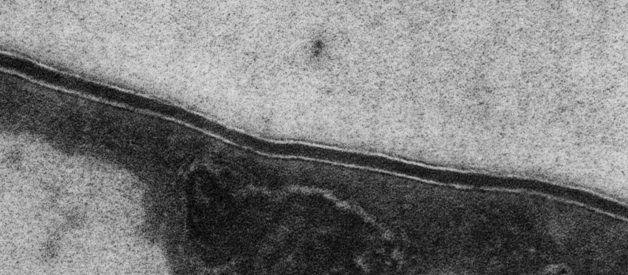
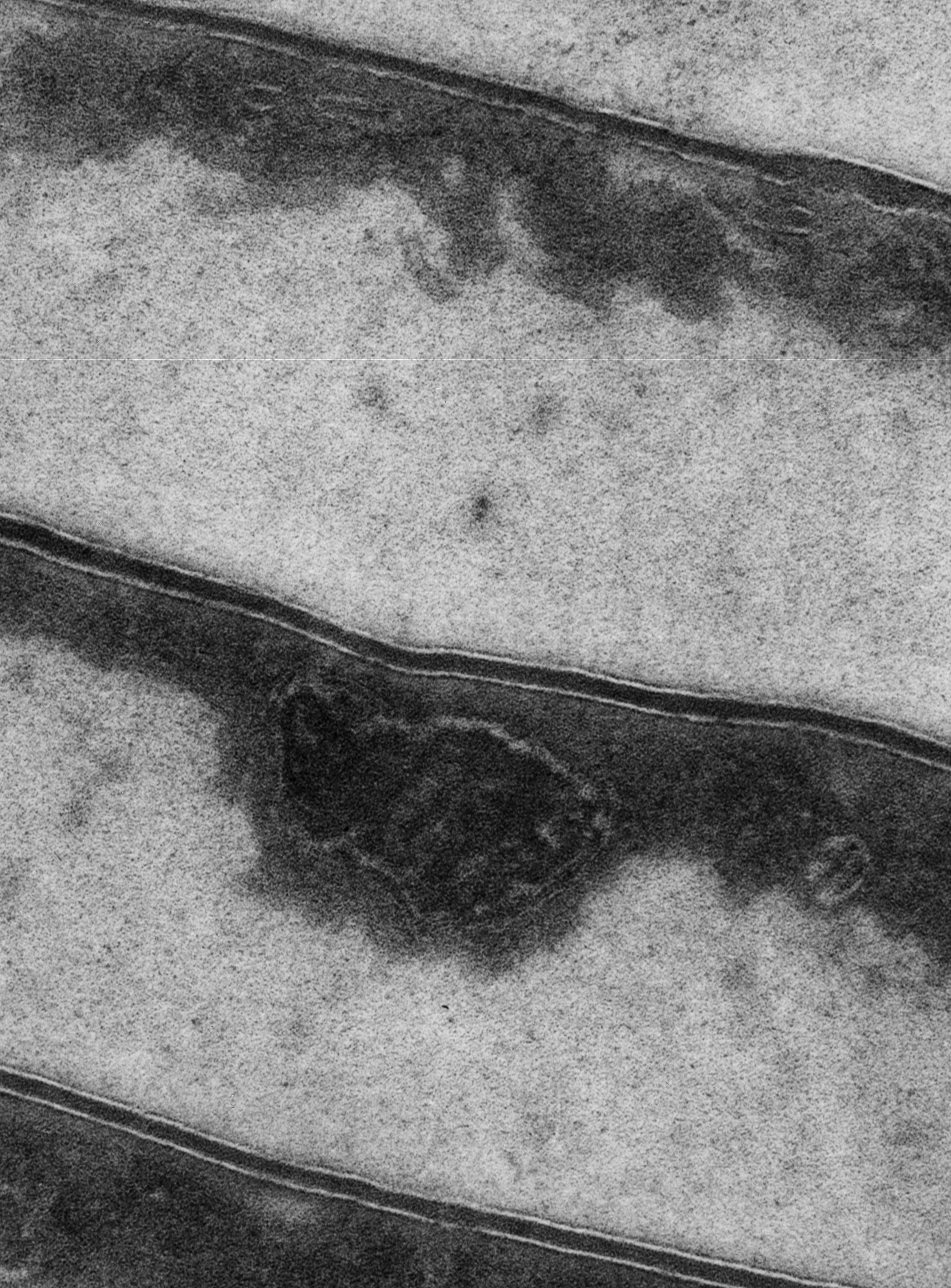 The cells of the cuticle are held together by the intercellular cement. L?Oral Research
The cells of the cuticle are held together by the intercellular cement. L?Oral Research
The hair also contains lipid components (3% of its composition). They are produced in the hair bulb from sterols, fatty acids and ceramides. Present essentially in the intercellular cement of the cortex and the cuticle, they provide the hair with a certain impermeability and ensure the cohesion of the hair fibre.
Other lipids come from the secretion of the sebaceous gland: sebum. Sebum is formed from mature sebaceous cells which have burst open and it mainly contains lipids (triglycerides, waxes, squalene, esterified cholesterol and free cholesterol). The most abundant triglycerides undergo partial hydrolysis by the bacteria that inhabit the scalp, Propionibacterium acnes and Propionibacterium granulosum. This hydrolysis liberates free fatty acids, di- and monoglycerides and glycerol. The fatty acids are characterised by carbon chains, even and uneven in number (from C11 to C19), with numerous unsaturated sections and branches. The waxes are esters of acids and long-chain alcohols. The waxes are apolar compounds which are metabolised by the flora and are little affected by oxidation.
 The surface of the scalp seen under a scanning electron microscope shows the hair shaft surrounded by drops of sebum. L?Oral Research
The surface of the scalp seen under a scanning electron microscope shows the hair shaft surrounded by drops of sebum. L?Oral Research
In the sebaceous gland, acetate molecules condense to form mevalonic acid, an isoprenoid isomer and squalene, a linear hydrocarbon formed from 30 carbon atoms. The sterols are esterified by the fatty acids. The squalene/cholesterol ratio in the surface hydrolipidic film is a mirror of the biological activities of the sebaceous glands and the epidermis. The lipid mixture which forms this film on the surface of the skin lubricates the hair and thus preserves the suppleness and shine of the hair. Being hormone dependent, the sebum can be produced in excess and the hair becomes greasy and heavy. On the other hand, if too little is secreted, the hair becomes dry, dull and damaged.