The company says it paused operations after an FDA notice, but customer demand remained high
 Credit: picture alliance/Getty Images
Credit: picture alliance/Getty Images
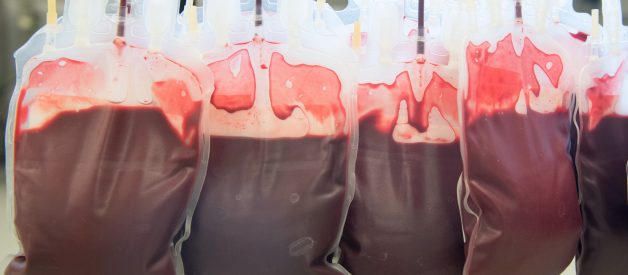
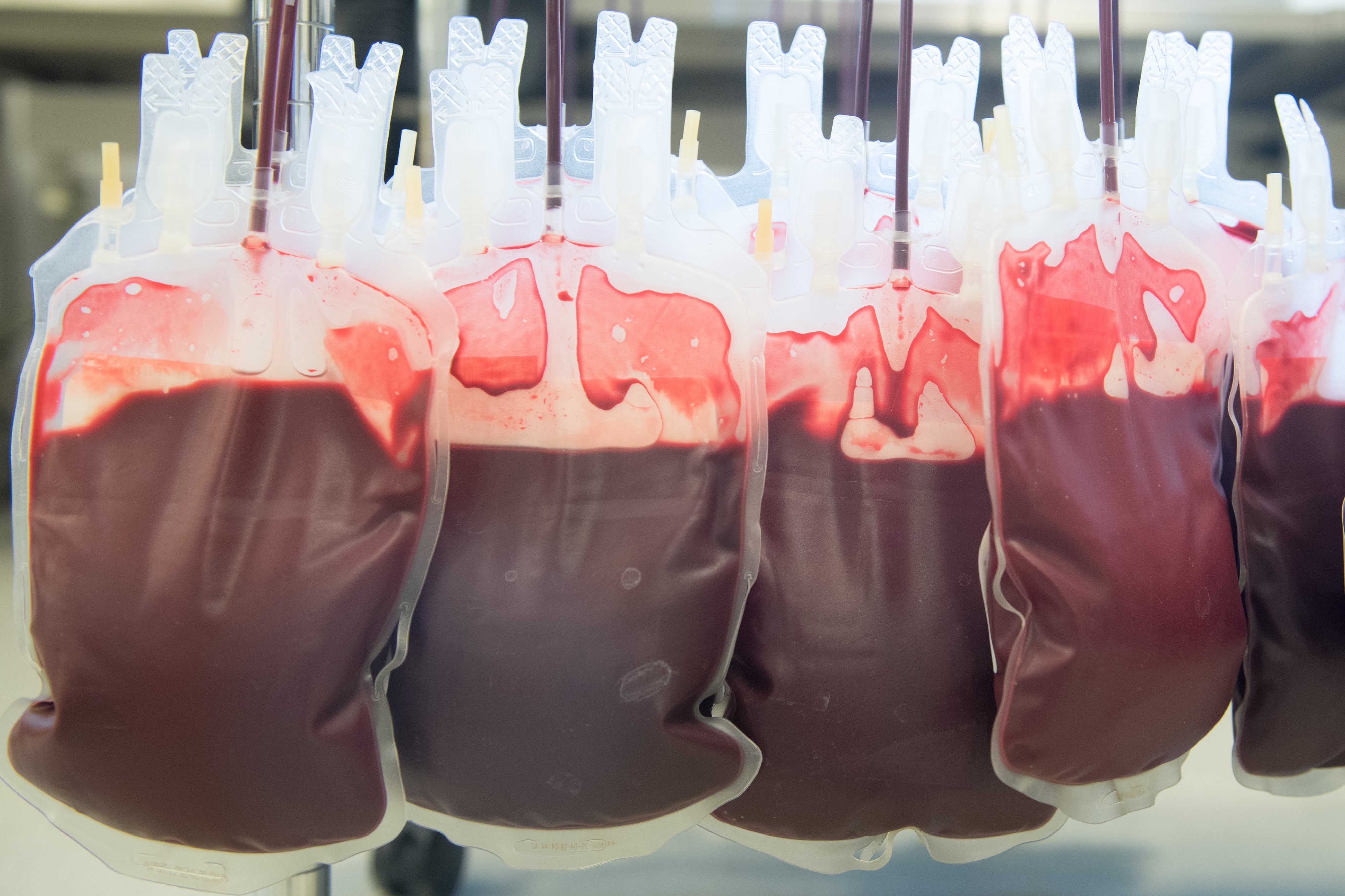
 Earlier this year, Ambrosia, the much-maligned California startup selling blood transfusions from young donors, stopped offering the procedure after the U.S. Food and Drug Administration issued a buyer beware, cautioning consumers against using the service. But now, according to Ambrosia?s CEO, the company is back up and running.
Earlier this year, Ambrosia, the much-maligned California startup selling blood transfusions from young donors, stopped offering the procedure after the U.S. Food and Drug Administration issued a buyer beware, cautioning consumers against using the service. But now, according to Ambrosia?s CEO, the company is back up and running.
Jesse Karmazin, the CEO and founder of Ambrosia, told OneZero in an interview that the company had resumed giving customers transfusions of plasma, the colorless liquid part of the blood, from young donors about a month ago. ?Our patients really want the treatment,? he said. ?Patients are receiving plasma transfusions from donors ages 16 to 25 again.? One-liter transfusions cost $8,000, and two-liter transfusions are $12,000.
In a pitch about Ambrosia at a 2017 conference on self-enhancement, Karmazin said, ?We?re a company interested in making you young again.? Plasma contains proteins that help the blood clot, and transfusions are often performed on patients to manage excessive bleeding, such as in trauma cases, and to treat clotting disorders like hemophilia. But experts say there?s no basis for using plasma to slow or reverse aging or age-related diseases, like Karmazin has claimed. Critics have blasted Karmazin?s transfusions as snake oil.
Controversy over the company reached a head in February 2019, when then FDA commissioner Scott Gottlieb issued a strongly worded statement warning consumers against such transfusions, saying there is ?no proven clinical benefit? of infusing plasma from young donors to mitigate, treat, or prevent aging, memory loss, or a host of serious medical conditions like Alzheimer?s. Ambrosia?s website claims that its transfusions have been found to ?produce statistically significant improvements? in biomarkers related to Alzheimer?s disease, cancer, and inflammation, and that customers have reported ?subjective improvements? in memory, sleep, and other areas.
?We?re alerting consumers and health care providers that treatments using plasma from young donors have not gone through the rigorous testing that the FDA normally requires in order to confirm the therapeutic benefit of a product and to ensure its safety,? said Gottlieb and Peter Marks, director of the FDA?s Center for Biologics Evaluation and Research, in the statement. They went on to say that some consumers are being ?preyed upon by unscrupulous actors? and that young blood transfusions could even be harmful.
The FDA did not go as far as issuing a warning letter, which the department does when drug or medical device companies violate federal regulations. Karmazin says the FDA did not contact him before or after issuing its statement in February and did not take any enforcement action against Ambrosia. Still, Karmazin says he stopped transfusing patients almost immediately after the FDA?s statement and put his business on hold ?under an abundance of caution.? He says he?s since had a number of communications with the agency but wouldn?t go into further detail.
?Our patients really want the treatment.?
In August, Business Insider reported that Ambrosia had shuttered following the FDA statement and Karmazin had started another business, Ivy Plasma, in its place. The Ivy Plasma website indicated that the company also planned to offer plasma transfusions for the same price as Ambrosia?s services, though the plasma was not sourced specifically from young people. But in his interview with OneZero, Karmazin said that Ivy Plasma was a short-lived rebranding effort to test customer interest in other products. The Ivy Plasma website now redirects to Ambrosia?s website. It turns out customers didn?t want transfusions from just anyone and one month ago, Ambrosia resumed operation.
?People really like the Ambrosia name and brand, so Ambrosia is going to continue,? he says. ?The resounding response from people wanting to sign up was, ?keep things the same.? So that?s what we?re going to do.?
Karmazin says he never closed Ambrosia entirely. The company was previously based in Monterey, California, and offered transfusions in at least two clinics, one in San Francisco and one in Tampa, Florida before February. It had plans to expand to other cities around the country. Now, the company is down to one clinic in San Francisco.
Karmazin is a graduate of Stanford Medical School but does not have a license to practice medicine and does not do the transfusions himself. Instead, he contracts with doctors to do the procedures. When asked, he would not name any doctors he works with or other Ambrosia employees. He says the company does not obtain blood directly from young donors but gets it from licensed blood banks in the United States. Karmazin says Ambrosia can legally provide transfusions ?off-label,? meaning that a doctor can prescribe a drug or treatment for something other than its approved use.
The FDA sees things differently. ?The FDA has not licensed or approved any plasma product obtained from young donors for any use,? the agency said in a statement provided to OneZero. Plasma units from young donors to treat patients, sometimes referred to as ?young plasma,? are drugs within the meaning of the Federal Food, Drug, and Cosmetic Act.? The FDA said it does not comment on specific firms.
Karmazin declined to say how many total customers Ambrosia has, but said people of all ages, from their thirties to nineties, some of whom are healthy, have received transfusions.
Karmazin ran a pay-to-play clinical trial from 2016 to 2018 to test the efficacy of Ambrosia?s procedure. Individuals were charged $8,000 to receive a similar transfusion to the ones he offers now. He has yet to publish any results.
He told OneZero that he has no plans to do further studies on the safety and efficacy of the Ambrosia treatment. ?This treatment is available now. Trials are very expensive, and they take a really long time,? he says. ?I?m comfortable with going ahead and offering this treatment commercially to patients.?