Water is normal in our everyday lives, but it?s also very weird
 Credit: fotografierende (adapted by author)
Credit: fotografierende (adapted by author)
Water, water everywhere
Water is so normal that it?s easy not to think about it.
It?s a liquid at room temperature. It?s transparent. It?s odourless.
We drink it, wash in it, swim in it.
We irrigate our fields with it, cool our factories with it, float ships on it. We skitter under the nearest shelter to avoid it when it falls from the clouds.
It can put out fires. It can dissolve salt, sugar and a whole number of other things in it.
We certainly can?t live without it. The majority of molecules in your body are water ? far more than any other kind. And yet if immersed in it for too long, we drown and die.
H2O
It?s possibly the most famous chemical formula there is: H2O.
 Credit: Booyabazooka
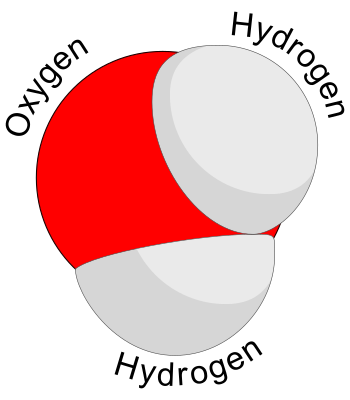
Credit: Booyabazooka
In chemical drawing, there?s a convention that oxygen atoms are red and hydrogens white.
For every atom of oxygen, there are two hydrogen atoms.
The atoms aren?t arranged in a straight line. Instead, they sit in a V shape. This is because oxygen has two points for bonding and two other hidden points all for itself.
Greedy oxygen atoms
All atoms have a nucleus at the centre which is positively charged. Whizzing around them is a cloud of negative electrons.
Oxygen is a hungry atom. It has more electrons than hydrogen. It loves to pull the electrons of its neighbours towards it too. Its greed makes it overly negative.
Hydrogen can?t resist this pull so when it?s attached to oxygen, it loses a bit of its hold on its negative electrons. It ends up a little bit more positive than if it were sitting all on its own.
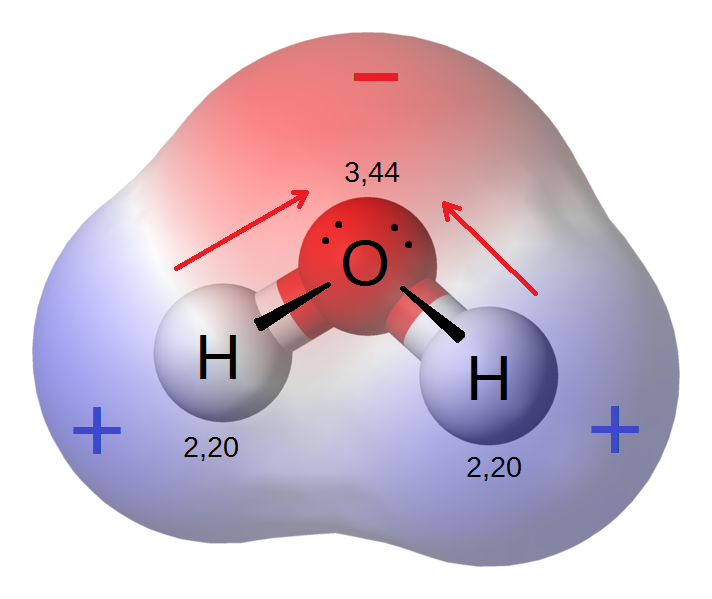 Credit: Riccardo Rovinetti
Credit: Riccardo Rovinetti
Poles apart
In water, this means the point of the V shape ? the oxygen ? is more negative than the tips where the hydrogens sit. The tips of the V are therefore a little positive.
The term for this is ?polar? because of the opposites contained at either end of the V. Polar molecules like water interact best with other structures that are also polar.
Opposites attract
Positive and negative attract each other.
When you put two water molecules together, the hydrogens are attracted to the oxygens. Add another water molecule and another and you quickly form a three dimensional network.
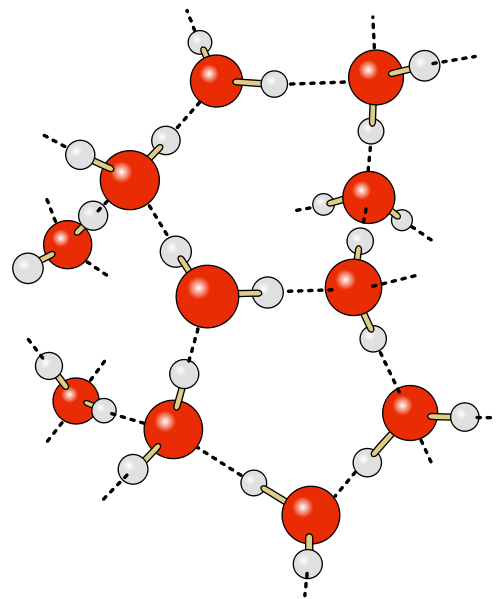 Credit: chris ? (vectorisation), Raimund Apfelbach
Credit: chris ? (vectorisation), Raimund Apfelbach
Water is again represented by red oxygens bonded to two hydrogens. The dotted black lines indicate the interactions between the positive and negative ends of the water molecules.
Other molecules of a similar sizes ? such as carbon dioxide, of greenhouse effect fame, are gases. Ammonia ? one nitrogen with three hydrogens attached ? is a gas on its own. To be useful to us, you can combine it with other substances to create solid fertilizer. Or you can make it into a cleaning fluid by dissolving it in ? you guessed it ? water.
Water has a cousin: H2S, hydrogen sulphide. Sulphur sits below oxygen in the periodic table, so H2S is a bigger molecule than water. You may be unfortunate enough to be familiar with it as the smell of rotten eggs. Despite being bigger and heavier, this molecule is a gas. Unlike water, the interactions between the molecules just aren?t strong. They are not enough to stick them together to make it a liquid at room temperature.
Stickiness
The strong attraction between water molecules gives it properties that are unusual, chemically speaking. For a start, it makes water especially sticky.
Water sticks to itself so well that it is a liquid over a large range of temperatures. Enough for it to practically cover our planet and enable life. Even when it?s cold, it freezes from the top down. The network of interactions between molecules becomes frozen in a configuration that ice is less dense than liquid water and floats on top. Weirdly???because it floats???ice makes a great insulating blanket for the water underneath, helping to keep it a liquid.


 Credits: Ashutosh Sonwani | Tanguy Sauvin | Min An
Credits: Ashutosh Sonwani | Tanguy Sauvin | Min An
This stickiness gives water other weird properties, too. It allows water to form beautiful little spherical baubles, such as those on a leaf. It lets water creep up inside a narrow tube or up the fibres of a paint brush against gravity. Little insects like pond skaters can walk on top of a calm pool of water without piercing it since the water molecules stick together so well that surface tension is very strong.
Is water wet?
It might seem like a simple question, but it isn?t all that easy to answer.
Is wetness a property of water or a description of how we experience it? If something is wet, does that mean it?s just interacting with water in some way?
A recent study summarised in the New Scientist estimated that you need at least six molecules of water for it to behave like a liquid. From this, we could say that you need at least six water molecules to make something wet.
The experience of water
OK, let?s put philosophical debates aside for a minute and decide on a definition for this discussion:
We experience water as wet. We can translate this by saying that an interaction with water means being wet.
If you drop some water on your skin the molecules will stick and you?ll be wet. If you spill water on your t-shirt, the fabric molecules draw water into it, even against gravity. The positives and negatives within the water molecules are attracted to the positives and negatives in the molecules of your hand or t-shirt. That?s why they get wet.
What about a plastic surface? That repels water because it?s made out of oils. But even if it?s repelling water, the surface is covered by it. It?s certainly experiencing water in some fashion.
 Credit: Snapwire
Credit: Snapwire
Why is water wet?
We?ve seen that the structure of H2O is polar. Water molecules attract other water molecules to form a network.
Being polar means it sticks well to other things, too, especially things that are also polar???like fabric or paper. Even if it doesn?t stick well to the surface, it covers the surface in droplets.
Wetness refers to the ability of water to stick to itself or something else.
Water is wet because, as we?ve seen, it is jolly sticky stuff.