Look back to literally anywhere in time where there are humans, and you?ll find fire. Fire has been an integral part of our society for ages, and thus it only makes sense that we?re able to understand what it is.

However, in a discussion ? or argument, depending on how you phrase it ? I got into with a friend the other day was asking what state of matter fire is. There are four fundamental properties:
- Gas
- Liquid
- Solid
- ?and plasma
It?s not a solid, so we can rule that out right off the bat, but it?s not really a liquid. Would we consider fire a gas? Not exactly. Probably not plasma, either. That?s when it comes to asking where we officially classify fire.
The first thing to do to officially break this down is to figure out what the major properties are of each of the states, and looking at which of those properties fire is able to qualify as. By doing this, we?ll be able to get a basic idea of where fire would make sense.
Finding Flaming Hot Properties
Well, let?s start by comparing fire to a gas. Most gases have no fixed shape or volume. Fire doesn?t really have a shape, and the volume expands or contracts depending on what you add to it (in this case, oxygen). So it?s a gas, right? Not quite ? if you put fire in a container, it?s not going to expand and fill the container in the same way as any other gas. This disqualifies it from constituting as a gas.
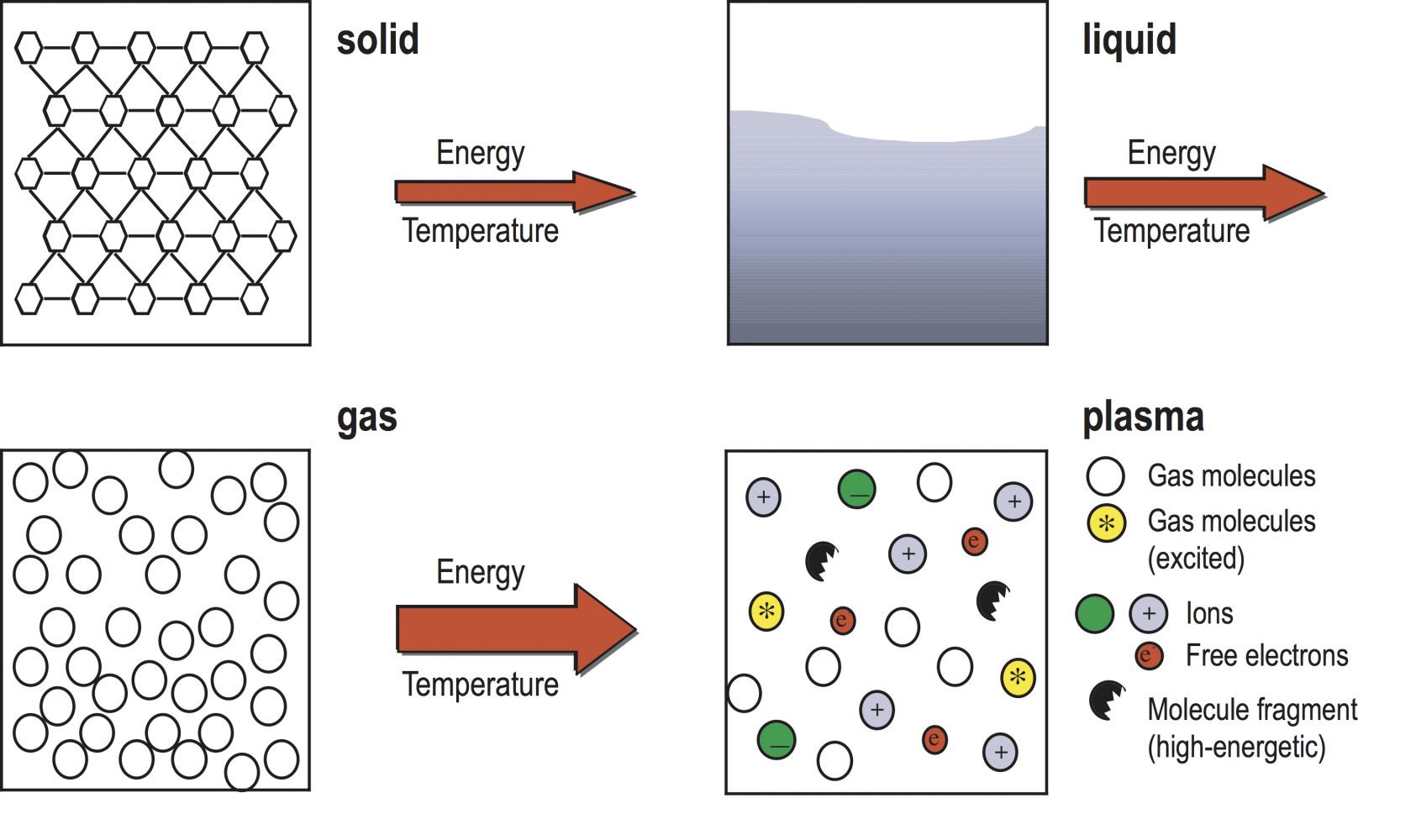 Some of the basic molecules and layouts of the states of matter.
Some of the basic molecules and layouts of the states of matter.
Next, we?ll check out a solid. That would mean it has a fixed shape, nice and stable. The biggest issue here is fire not having this main, integral property. If you try and pick it up, it will burn through your hand (likely), rather than keeping the same shape you found it in. It moves if you blow on it. Fire isn?t a solid, either.
Up next: a liquid. Liquids don?t have to conform to a particular shape ? meaning it?s looking good for fire ? but they also do have a fixed volume, something the fire undoubtedly lacks. So it isn?t really a liquid either.
If you look at fire?s atomic structure, as well, it?s not exactly able to form structures like the other forms of matter, not under the influence of a magnetic field. That means you?ll never be able to have a fire electromagnet ? sorry to disappoint. In addition, fire will cease to exist if it isn?t continually fed ? unlike all other forms of matter, which are able to continually exist.
Lastly, laws of physics don?t permit extracting more energy out of a substance than you put in. That?s like getting more money out of an already-empty debit account; it won?t work. There?s overwhelming evidence that fire isn?t any of the aforementioned states of matter. So what is it?
There?s one final state of matter, and that?s plasma.
The Fundamentals Of Plasma
So this is fire ? well, not quite. Before the discovery of plasma, it was widely agreed that fire was its own element, because it didn?t conform to any of the categories which most matter fits into. However, the reason fire gets this classification is because it?s most like plasma. It?s not a perfect match, but it?s as close as science can come at this moment.
Plasma has a quality where electrons are more free-roaming around the nuclei, rather than fixed in particular positions like in the other three kinds of matter. It?s more of a particle cloud than it is any other type of matter, and although plasma does bear some resemblance to gas, it?s still quite different.
TL;DR ? What REALLY Is Fire?
- There are four states of matter. These states of matter are used for classification of everything, and it?s up in the air where fire falls.
- Fire doesn?t fall into gas, because it doesn?t expand in the same way gas does.
- Fire doesn?t fall into liquid, because it doesn?t have a fixed volume.
- Fire doesn?t fall into solid, because it doesn?t have a fixed shape.
- Thus, fire is currently considered a plasma. This was after countless years of considering fire to be its own element. Plasma makes the most sense because it has free roaming electrons, which isn?t seen in any other state of matter ? but is seen in fire.
Thank you so much for reading this article! It was certainly fun to write ? and I hope you got the chance to learn something too! If you?d like to talk, please email me at [email protected] or find me on LinkedIn under Amelia Settembre. Always down to learn anything! 🙂

