I buy my toothpaste from overseas through eBay. Wonder why?
Everyone knows about fluoride in dental products. If you?ve been living under a rock, here?s a nifty article to educate you on why your toothpaste contains fluoride.
Fluoride has been in use for preventing dental caries since the 1940s. Depending on which articles you read, fluoride either works wonders or doesn?t work at all or, worse, it?s a globalist agenda to curb population growth.
I won?t make a conclusion as to whether fluoride is actually effective in preventing dental caries, but it is clear that the occurrence of caries has steadily decreased in developed countries. Take that for what you will.
Maybe fluoride works, or maybe it doesn?t. But don?t you think that there must have been some advancement in dental hygiene since the 1940s? That was generations ago, and we must have discovered more ways to prevent tooth decay.
In fact, we did. And few even know about it.
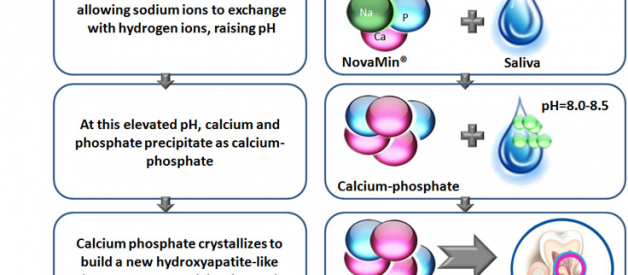
Calcium Sodium Phosphosilicate is a bioactive glass compound invented in the 1960s for the purpose of bone regeneration for troops wounded in combat. It was later adapted into dental applications through research funded by a Florida company called USBiomaterials. In 2003, USBiomaterials spun off its dental research into a VC-funded startup called NovaMin Technology, Inc.
CSPS is more commonly known by the brand name NovaMin.
According to an archived version of the now defunct NovaMin website:
The origins of NovaMin go back to a chance encounter between a materials scientist and an Army Colonel and their conversation about helping to repair the shattered bones of soldiers who were battle wounded. That conversation led to a revolutionary bone repair material. After over a million patients benefitted from that creation, researchers found a way to adapt the same technology for renewing the vitality of teeth. Thus, NovaMin was born.
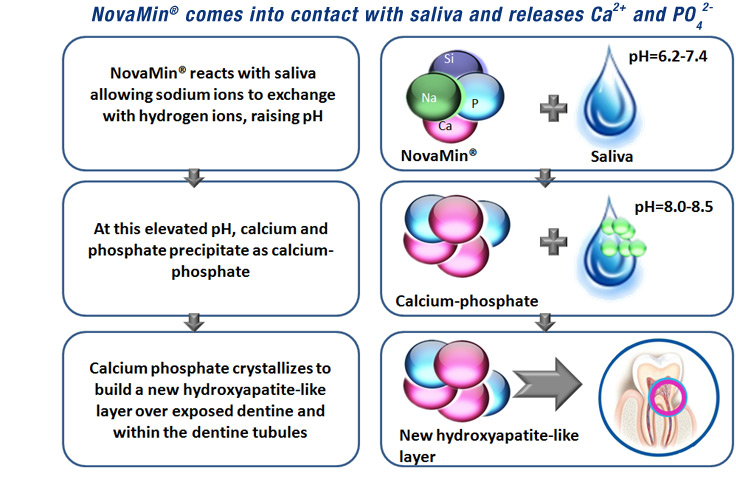
When exposed to water and saliva, NovaMin forms hydroxyapatite-like crystals that occlude exposed dentin tubules in teeth. Essentially, it compensates for damaged enamel by creating a temporary repair, both relieving tooth sensitivity and preventing new caries from forming in exposed tubules.
 Courtesy: GlaxoSmithKline plc
Courtesy: GlaxoSmithKline plc
Studies have found that NovaMin is effective in treating dentin hypersensitivity, treating caries(the study was carried out on artificial caries), and even enhancing the effectiveness of fluoride by 50%.
NovaMin might be particularly beneficial to the elderly. According to a 2007 article from The Guardian:
Researchers have found fluoride ceases to be as effective with older people. That?s because the elderly have more difficulty generating the large amounts of saliva ? loaded with calcium and phosphate ? necessary to combine with fluoride to resist the demineralisation of teeth.
NovaMin would provide the missing minerals required for the process of protecting teeth with fluoride.
By the way, leave a comment if you can figure out the part of that article which made me laugh hysterically.
The first toothpaste to include NovaMin was by 3M/Omni called ?SootheRX?, available by prescription only. NovaMin later began to appear in OTC products such as Oravive, Burt?s Bees, and Dr. Collins Restore Toothpaste.
In December 2009, the pharmaceutical giant, GlaxoSmithKline plc, acquired NovaMin Technology Inc. in a deal worth $135 million.
It wasn?t long before NovaMin began disappearing from upwards of 17 toothpaste brands. Although I don?t know precisely when GSK ended its supply of NovaMin to other brands, I do know that the last time I spotted a tube of toothpaste at my local drug store with Calcium Sodium Phosphosilicate in the list of ingredients was in 2012. (Burt?s Bees Natural Toothpaste)
I would understand GSK pulling NovaMin from other brands in order to promote the use in its own brand of toothpaste, Sensodyne, but then why would it also stop selling the version of Sensodyne that?s ?Powered by NovaMin? in the United States?
 Sensodyne Repair & Protect toothpaste, European packaging(top) and US packaging(bottom)
Sensodyne Repair & Protect toothpaste, European packaging(top) and US packaging(bottom)
Rumors are abound among the few out there who have been asking the same questions that I am. Some say that the FDA banned NovaMin, but I?ve found no evidence to support this. One is likely to entertain the idea that GSK was paid off by the dental industry to discontinue NovaMin. I have no evidence to support that claim, either.
Perhaps GSK wasn?t paid off, but simply discontinued the product because its effectiveness could impact revenue from its other dental products it sells to dentists? I mean, who?s really going to need ongoing treatment bonding for cavities if their teeth are already being protected by NovaMin? Just a thought.
What I do know is that while NovaMin is completely unavailable in the US(as far as I?m aware), it?s widely available in other countries under GSK?s own brands Sensodyne and professional product NUPRO, a cleaner for dental hygienists. The product website for NUPRO shows that potential buyers in the United States need not apply.
Why would GSK market a ?safe? toothpaste in countries around the world but not in the United States?
GSK does not have a monopoly on bioactive glass compounds, although they have control over what contains NovaMin since they own the patent. Other products have popped up such as BioMin, a toothpaste that contains Fluorocalcium Phosphosilicate. But again, this is another toothpaste that is not available in the United States.
Why are there no bioactive glass toothpastes being sold in the United States?
These are questions I want answers to.
UPDATEI came across a new mouthwash available in the USA by Listerine that works similar to NovaMin but uses Dipotassium Oxalate Monohydrate to occlude dentin tubules. I don?t know if any studies show that DOM has any effect on caries.
UPDATE 2(8/31/2019)My quest for answers is over! I have just received some information from someone connected to GSK, the patent-holder of NovaMin technology and producer of NovaMin toothpaste.
As much as a conspiracy by Big Toothpaste to keep NovaMin from the American public would have been compelling, the reason that you currently don?t find toothpaste containing NovaMin in the USA is fairly simple.
According to my source, GSK desperately wanted to market Sensodyne Repair and Protect w/ NovaMin, which is GSK?s flagship premium brand around the world, in the USA. But they ran into regulatory issues.
In the USA, toothpaste is regulated as a drug, whereas in the EU it?s regulated as a cosmetic. Drug labeling and advertising is heavily restricted, and rightfully so.
If GSK were to make therapeutic claims about Novamin in their toothpaste, GSK would need FDA-approved evidence to back up those claims. Running the required studies is expensive. The projected increase in revenue could not justify the costs.
This is why products with NovaMin are available elsewhere in the world except the United States. I assume the reason that products containing NovaMin were once available in the USA may be that they did not market the presence of NovaMin as an ingredient; Burt?s Bees toothpaste listed calcium sodium phosphosilicate in its ingredients, but made no mention of it or NovaMin anywhere else on its packaging.
As of the time of this update, Sensodyne toothpaste with NovaMin is now available on Amazon to buyers in the USA, directly from Sensodyne/GSK.