Inversion of configuration usually happens when an organic compounds undergoes Nucelophilic substitution reaction by SN2 mechanism.
A Nucleophile (Electron rich species having affinity towards electron deficient centre) can attack the Stereocenter in two ways, like it can attack from frontside or from backside.
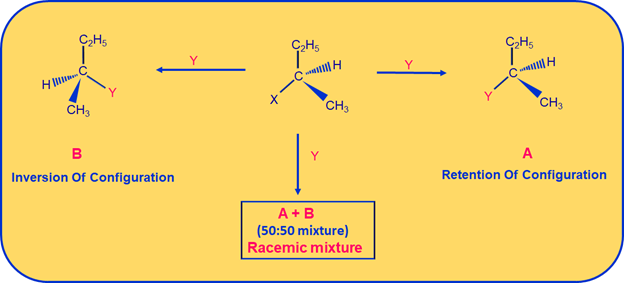
In frontside attack, the nucleophile attacks the stereocenter from the same side of the leaving group. This results in Retention Of Configuration. Retention of configuration means it is the preservation of integrity of the spatial arrangement of bonds to an asymmetric centre during a chemical reaction or transformation. It can be well understood with an example given below:
 Retention Of Configuration where Nucleophile Y- is attacking the Stereocentre from the front side of leaving group X-
Retention Of Configuration where Nucleophile Y- is attacking the Stereocentre from the front side of leaving group X-
In the backside attack, the nucleophile attacks the stereocenter from the opposite side of the leaving group. This results in formation of the product having Inversion Of Configuration.
Look at the following equation to get an idea about Retention Of Configuration & Inversion Of Configuration occurring in case of an organic molecule undergoing nucleophilic substitution reaction.
 Equation Depicting Both Retention & Inversion Of Configurations
Equation Depicting Both Retention & Inversion Of Configurations
Here halogen is substituted by the incoming nucleophile ?Y? attacking the stereocentric Carbon from:
a) frontside resulting in product having Retention Of Configuration as seen in structure A &
b) on the other side ?Y? is attacking from the backside resulting in product having Inversion Of Configuration as seen in structure B.
Since Inversion Of Configuration occurs by SN2 reaction let us understand it in depth.
Nucleophilic Substitution-Bimolecular reaction mechanism (SN2)
This type of nucleophilic substitution reaction follows a second order kinetics, i.e., the rate depends upon the concentration of both the reactants-the nucleophile and the haloalkane.
Order = 1+1 = 2 (sum of power of concentration terms in rate law)
Some important features of SN2 mechanism
1.Reaction gets completed in a single step itself and that step itself will be the Rate Determining Step. Hence it belongs to 2nd order kinetics.
2.Inversion of configuration occurs.
3.Carbocation formation does not occur. But involves the transition state formation.
4.Primary alkyl group & non-polar solvent favours SN2 mechanism.
Case Example:
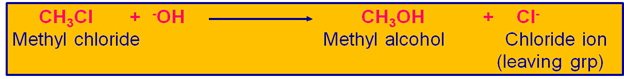
Let us take an example of organic compound, to understand this mechanism of SN2 which results in Inversion Of Configuration. The reaction between Methyl chloride (CH3Cl) & the attacking nucleophile ? the Hydroxide ion
(-OH) yields Methyl alcohol (CH3OH) & this reaction occurs by SN2 mechanism & belongs to second order kinetics.
 Reaction Equation Of Conversion Of Methyl Chloride To Methyl Alcohol
Reaction Equation Of Conversion Of Methyl Chloride To Methyl Alcohol
Rate = K [CH3Cl] [-OH]
Order = 1+1 = 2
 Hydroxide Ion Attacking The Partially +vely Charged Carbon Atom
Hydroxide Ion Attacking The Partially +vely Charged Carbon Atom
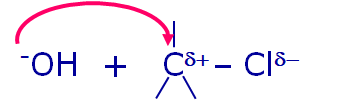
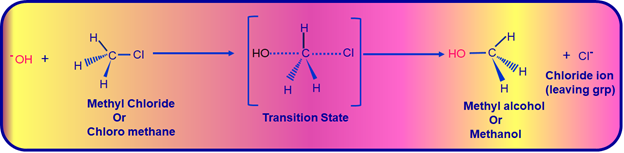
Here the Hydroxide ion (-OH) the incoming nucleophile attacks the partially positively charged Carbon center (the Carbon bonded to halogen atom will have partial positive charge on it due to difference in the electronegativity between Carbon atom & Halogen atom) from the opposite side of leaving group (out going group- in this case the Chloride ion Cl-). When this happens the Carbon-Halide (C-Cl) bonds is partially broken & the Carbon-Hydroxide (C- -OH) bond is partially getting formed. Both these reactions occurs simultaneously & this state of Carbon atom is called the transition state. In this transition state Carbon atom is bonded to 5-atoms around it making Carbon highly unstable. It is stabilized as the reaction progresses by the complete breaking of Carbon-Halide (C-Cl) bond as it becomes weak. Now, the halogen (Cl) leaves as Chloride ion (Cl- which is the leaving group) thus forming more stable Methyl alcohol or Methanol (CH3OH). Since the attack of the Nucleophile (-OH) has taken place from the opposite side of the leaving group, we observe the product formed will have inversion of structure when compared with the reactant Methyl chloride (CH3Cl) as shown in the equation below:
 SN2 Mechanism Involved In Converting Methyl Chloride To Methyl Alcohol
SN2 Mechanism Involved In Converting Methyl Chloride To Methyl Alcohol
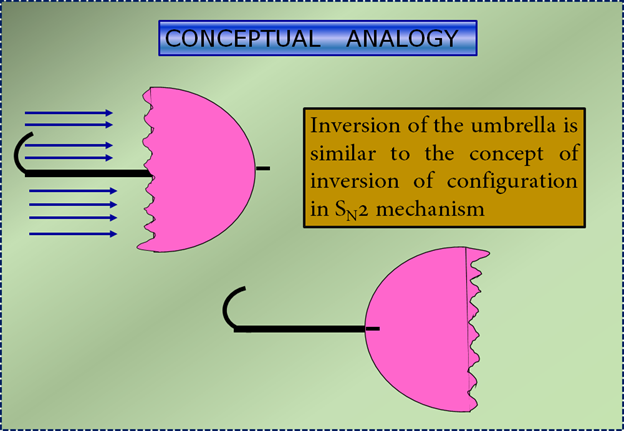
As the reactions between attacking Nucleophile (-OH) and Carbon starts, their bond strengthens & when this happens the configuration of Carbon atom under attack inverts in the same manner as an umbrella is turned inside out when caught in a strong wind. This process is called Inversion Of Configuration as can be seen the above equation.
Here is an conceptual analogy SN2 mechanism occuring like that of an umbrella turning inside out- Inversion Of Configuration.
Do you Like this? Do clap
Then Follow BIC at :
Follow BIC on Youtube: https://www.youtube.com/user/pucbic
Follow BIC on Facebook : http://facebook.com/bicspuc
Follow BIC on Instagram :https://www.instagram.com/bicpuc1/
![Rate = K [ R-X] [-OH]](https://911weknow.com/wp-content/uploads/2020/09/rate-k-r-x-oh-5-624x275.png)

