Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory.
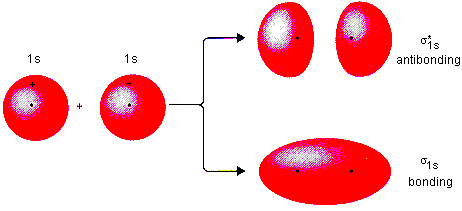
Valence Bond Theory proposes that electrons are localized between two atoms. On the other hand, Molecular Orbital Theory visions the electrons of a covalent bond to be delocalized over the entire molecule. Put simply, the valence electrons are not confined to individual bonds. Rather, we are considering the weighted linear sum of all atoms in a molecule.
The key is to first identify what molecule they are asking you to draw and then determine which of the following categories it belongs to. If you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw it.
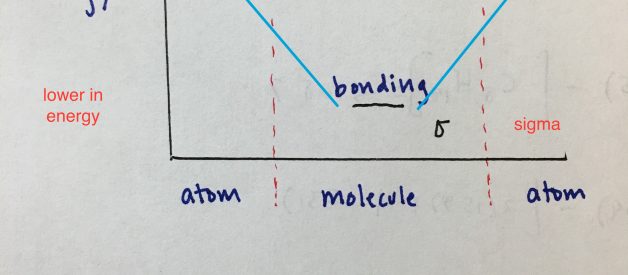
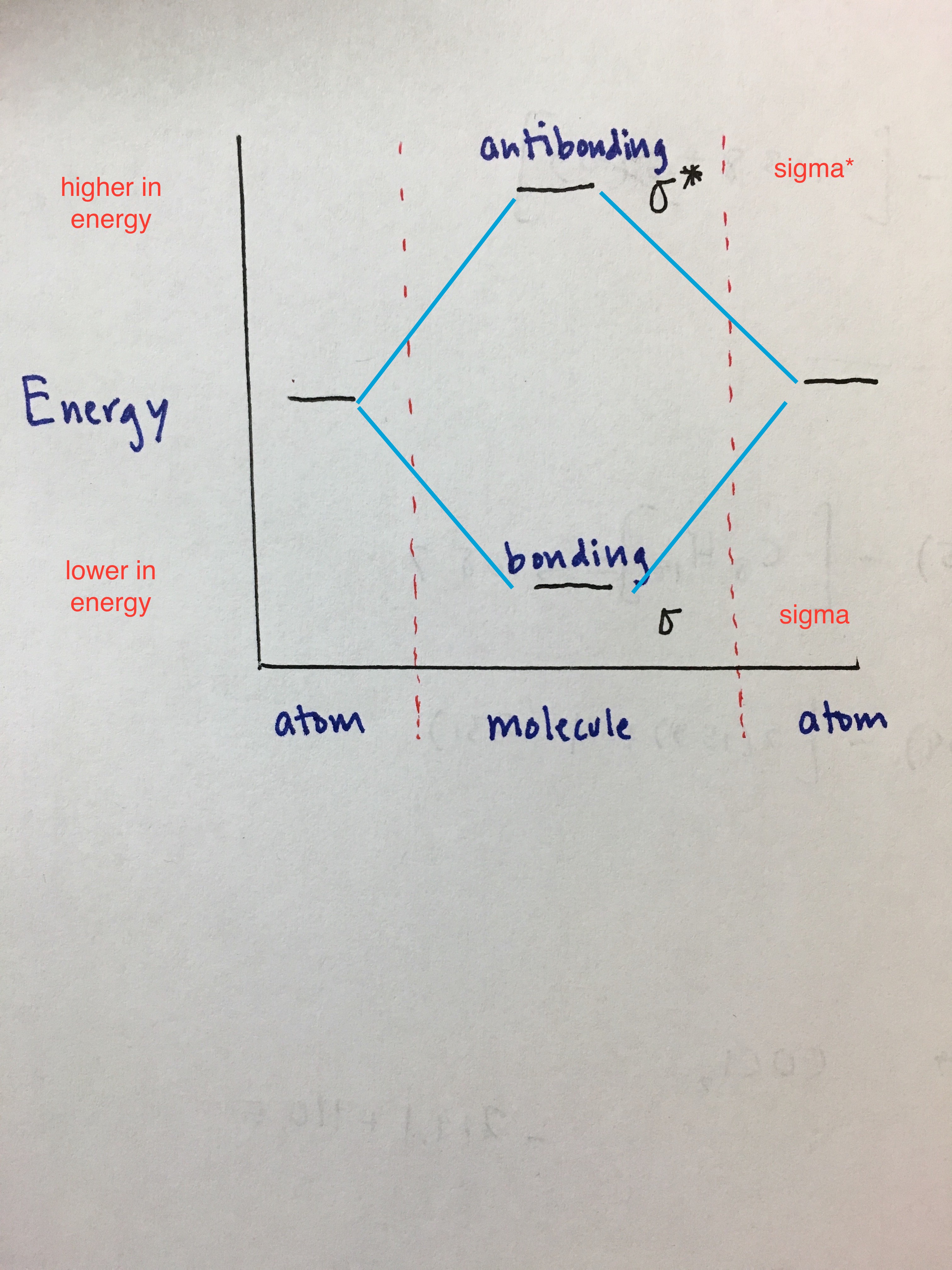
Basic Skeleton
Energy is on the y axis. You can see that the antibonding orbitals are higher in energy and bonding orbitals are lower in energy. The regions to the left and right side of the dashed lines are atomic orbitals. Inside the dashed lines are the possible molecular orbitals they are capable of forming.
Things to remember:
- if you have ?n? number atomic orbitals, you will form ?n? number molecular orbitals
- consider only valence electrons (only ones that are relevant in covalent bonding)
- place electrons with parallel spins and separate orbitals if possible (minimizes electron repulsions)
Homonuclear Diatomic Molecules
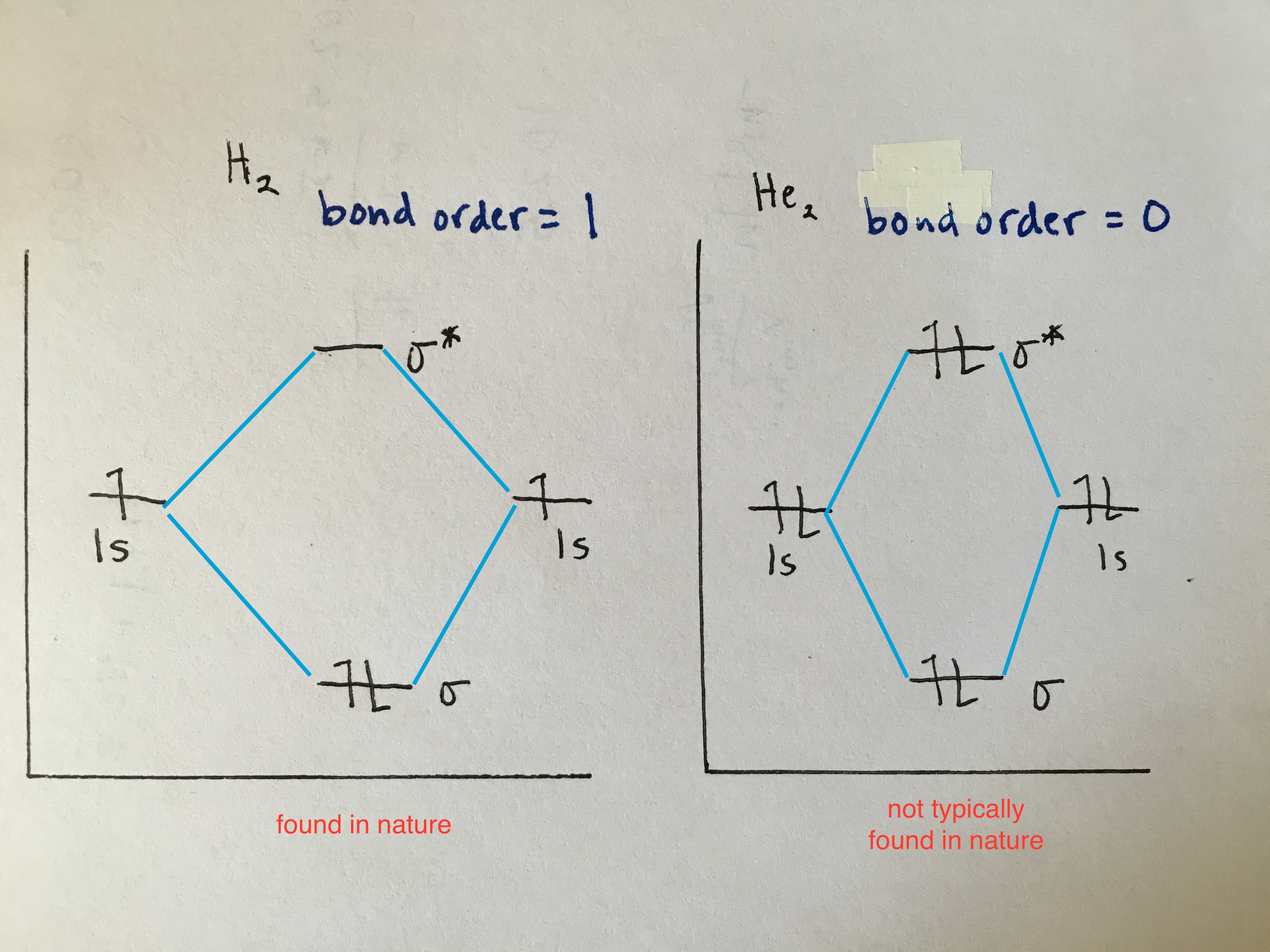
1st period(elements H-He)
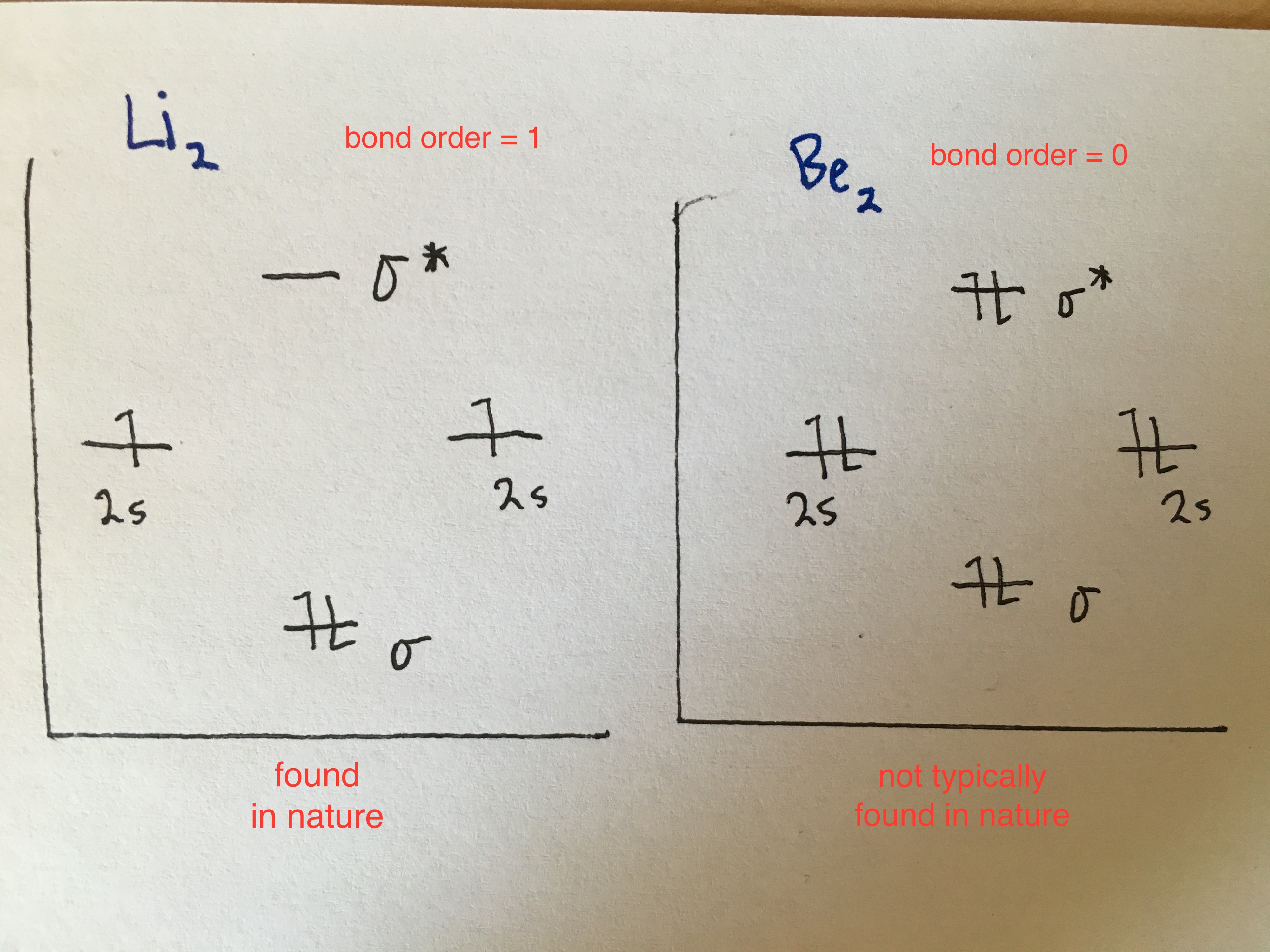
2nd period
Li-Be
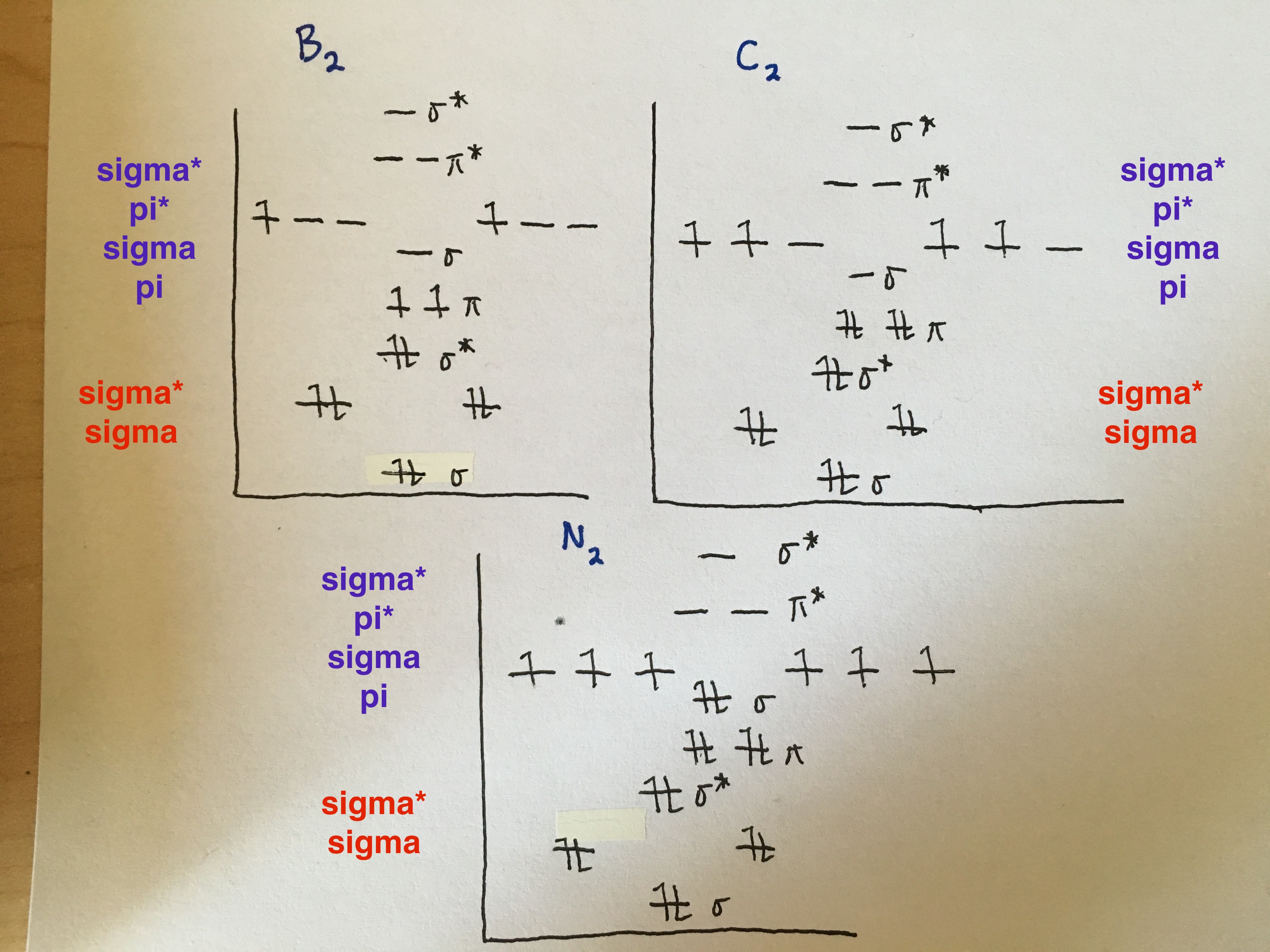
B,C,N- note difference in order of p orbitals from O,F,Ne
O,F,Ne- note difference in order of p orbitals from B,C,N
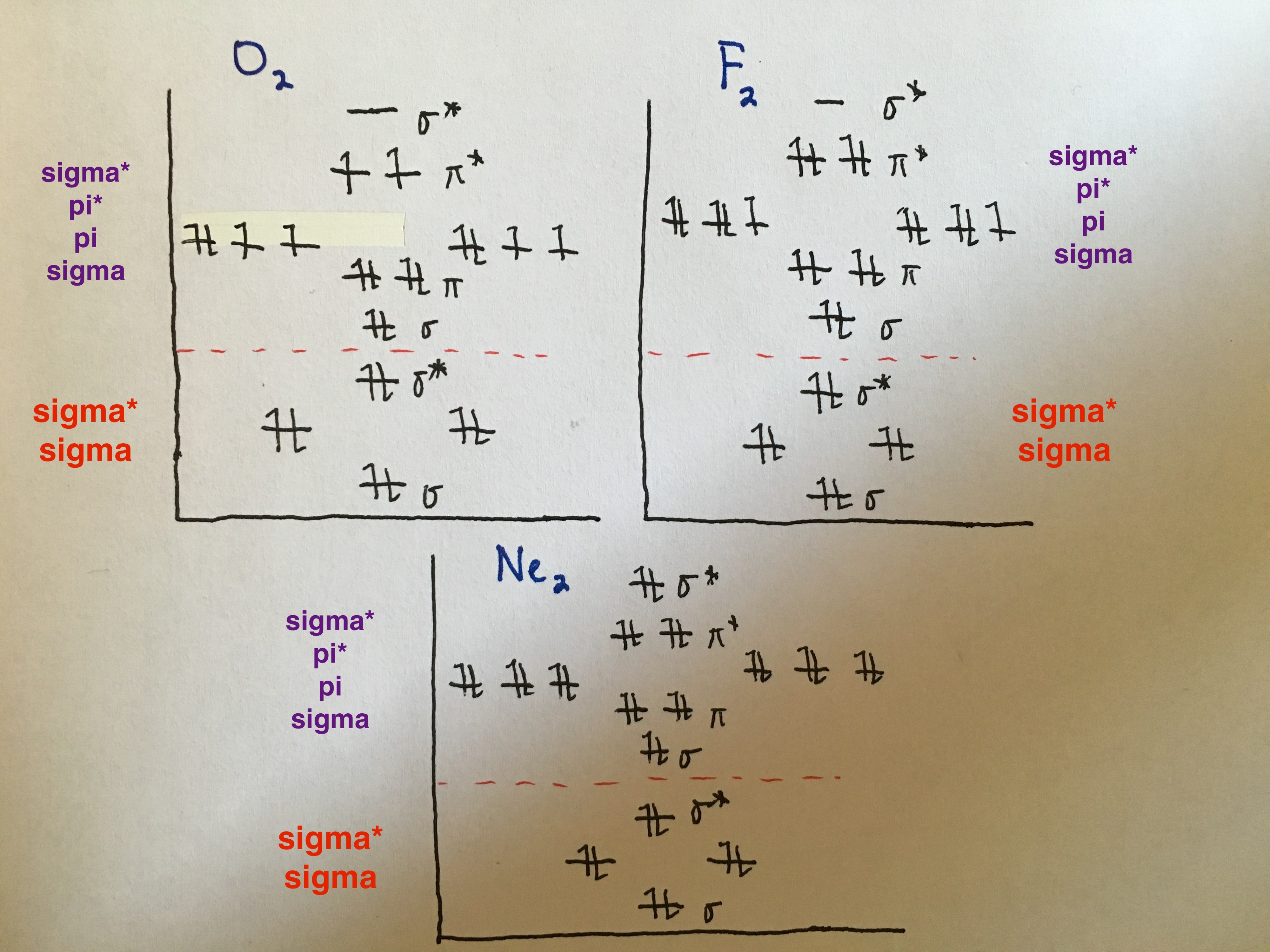
Note: when the 3 p orbitals (x,y,z) interact, they form 4 pi bonds and 2 sigma bonds

Heteronuclear Diatomic Molecules
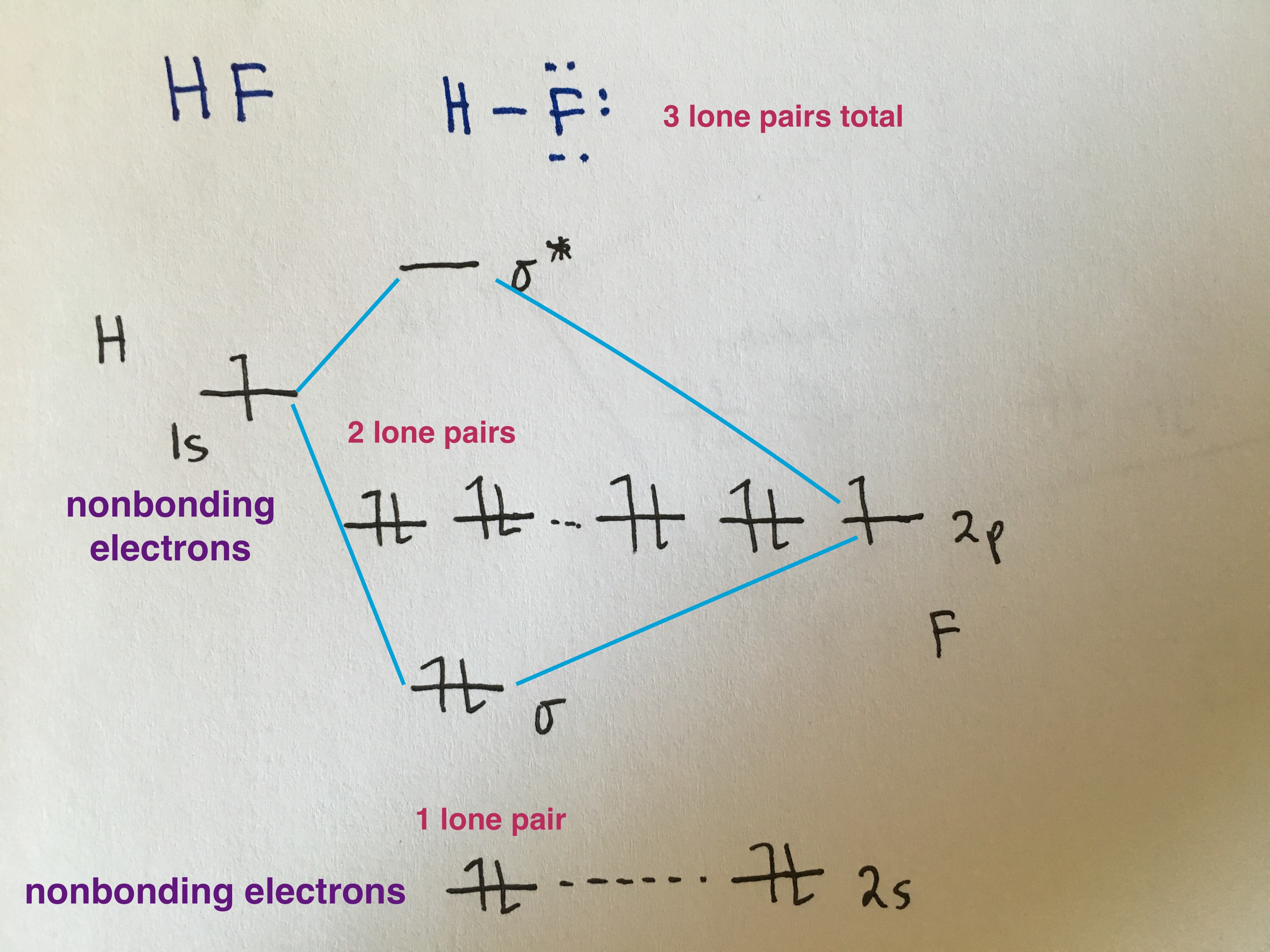
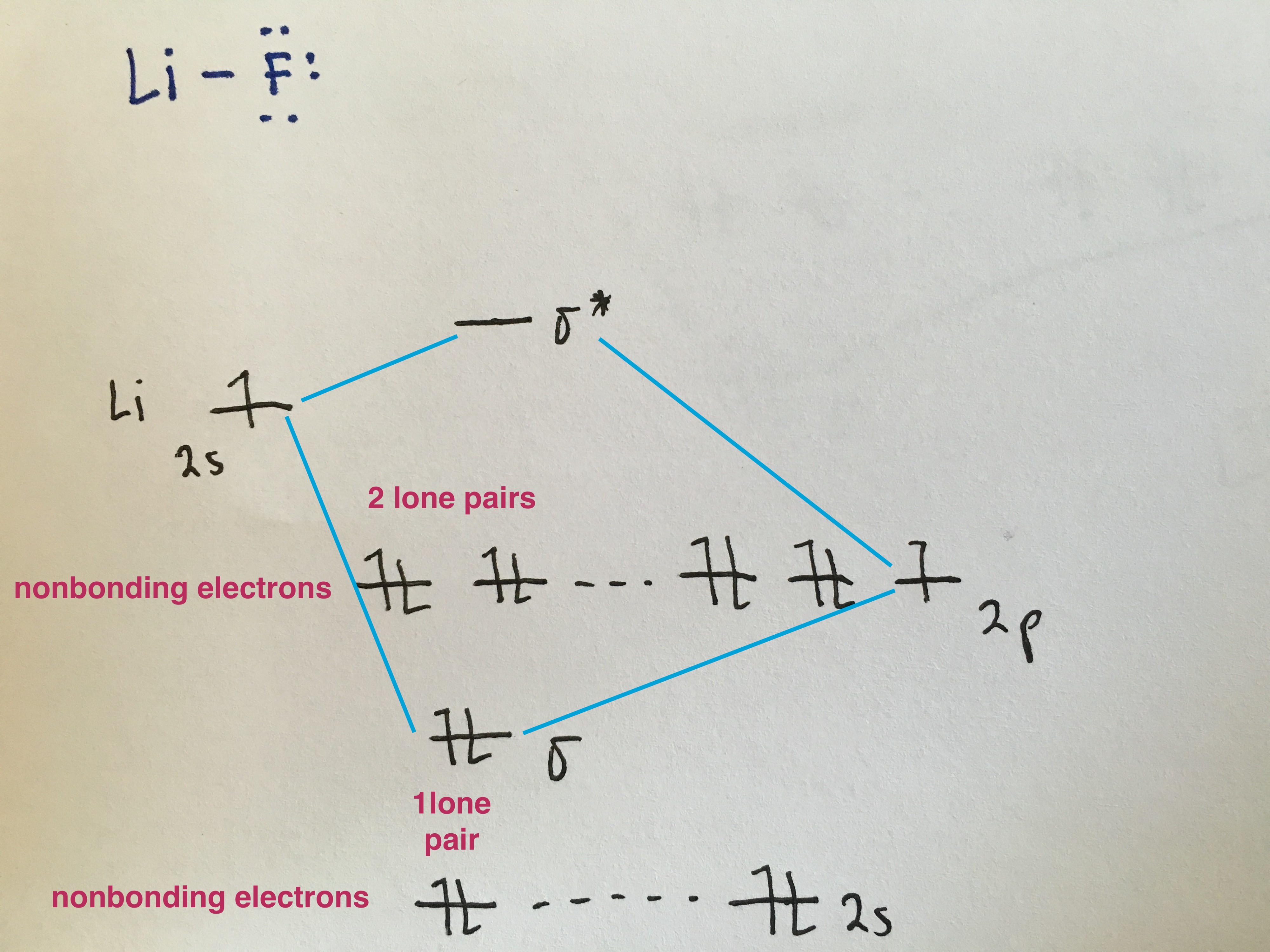
Because the electronegativity of the two atoms are unequal, the molecular orbital diagram will no longer be symmetric. Instead, the more electronegative element is drawn lower in energy and contributes more to the bonding orbital. And the less electronegative element is drawn at a higher energy level and contributes more to the antibonding orbital.
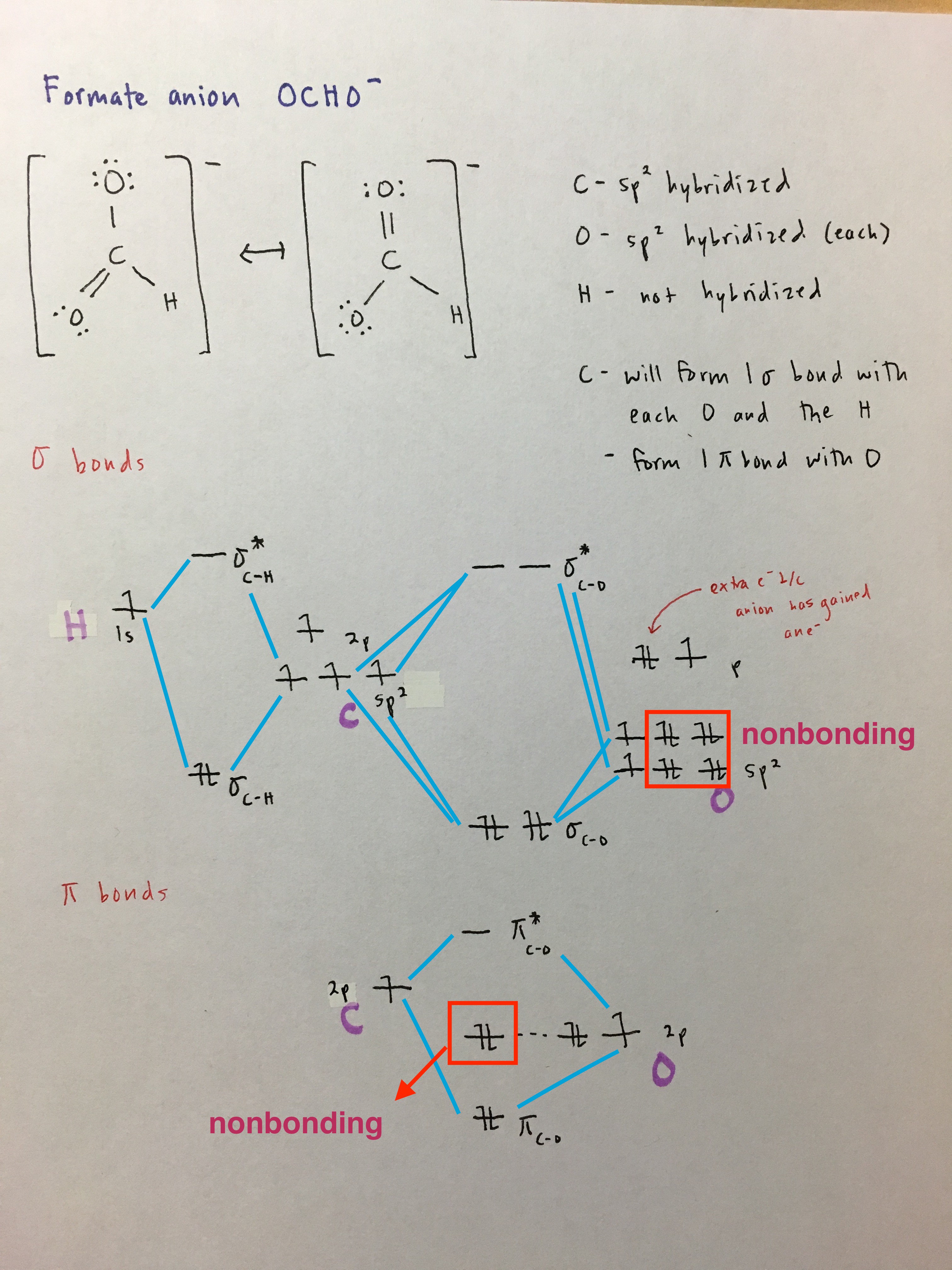
Polyatomic Molecules- trickiest
- draw Lewis structures (and resonance structures if possible)
- identify what type of bonds will be formed (hybridized/nonhybridized, sigma/pi)
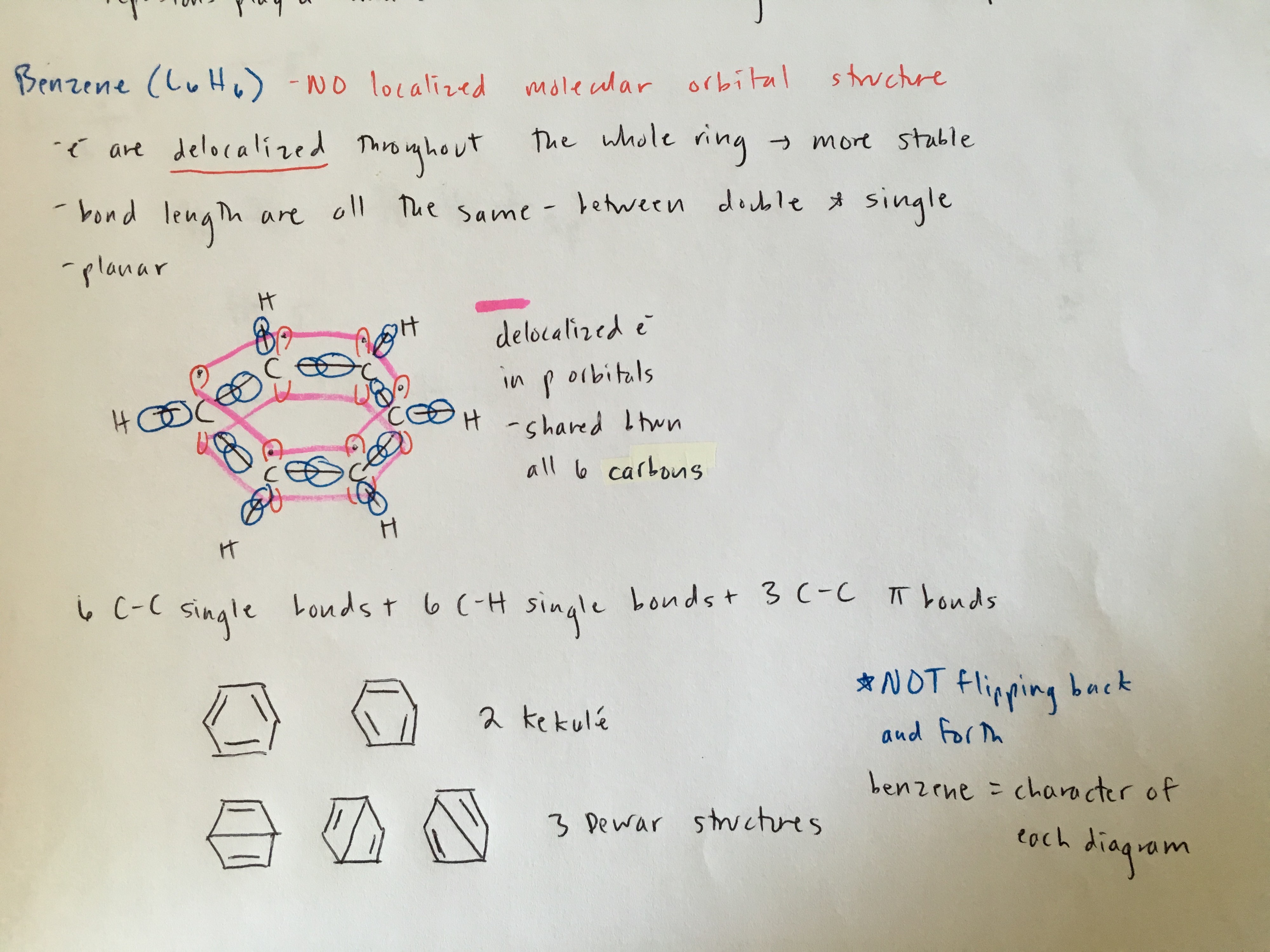
Benzene is a special type of molecule in which its 6 p electrons (1 electron from each carbon) is delocalized over the entire molecule. This leads to a more stable aromatic structure.