?Early to bed and early to rise makes a man healthy, wealthy, and wise.? ? Benjamin Franklin
If you enjoy my work, consider becoming a PATREON. This helps to support the content I create for you and others to enjoy. Thank you!
Recent evidence from some rigorously controlled feedings trials suggest that we may have to include ?early to dine? inside the contents of this oft-referenced quote.

This article was updated on 7/30/2019 with some new data from a brand new eTRF study (scroll to study #2 to see results of the second study).
More diet trials(s) I know ? CR, TRF, ADF, IF? the plethora of abbreviations we have for different ?types? of diet regimens (I don?t like to use the term ?diet? for these ? as they?re much more lifestyle changes/ways of eating) is astounding. The one thing they have in common is the search to improve human health and prevent (end) disease.
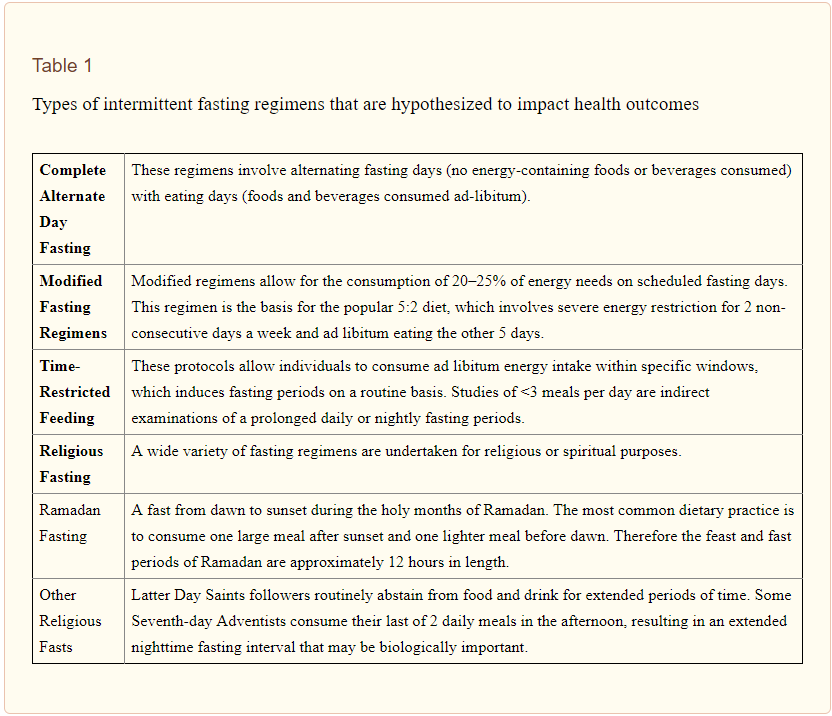 Some of the variations of Intermittent Fasting (IF) in the Literature. Source: J Acad Nutr Diet. 2015 Aug; 115(8): 1203?1212.
Some of the variations of Intermittent Fasting (IF) in the Literature. Source: J Acad Nutr Diet. 2015 Aug; 115(8): 1203?1212.
We may be getting closer to realizing the optimal, not composition, but ?structure? of eating that seems to suit metabolic health the most. Time restricted feeding (TRF) ? the up and coming star in the world of meal timing.
The simplicity of TRF makes it compelling ? just limit your food intake to a certain ?window? during the day (ideally, 4?10 hours, allowing for a 14?20 hour fasting window). The simplicity of TRF also makes it vague, not to mention complicated. We know we should eat within a ?window? ? but when should this window start and end? Should we skip breakfast and eat a larger lunch and dinner later in the day? Eat breakfast and lunch, but skip dinner?
In humans, four pilot trials of TRF have been conducted to date. Surprisingly, the results of TRF in humans appear to depend on the time of day of the eating window
From a biological perspective (and for a quick primer) ? theories point to an earlier feeding window benefiting health due to how our circadian rhythms are programmed. Eating ?in line? with our internal clocks is optimal for a way to improve responsiveness of our body to food, and match intake with the ?expectations? of our circadian rhythms ? our internal biological clocks present in every tissue. Eat when our rhythms are primed for food intake, when hormones for energy metabolism are primed, and we avoid subjecting our bodies to undue oxidative stress, glucose spikes, and other metabolic derangements.
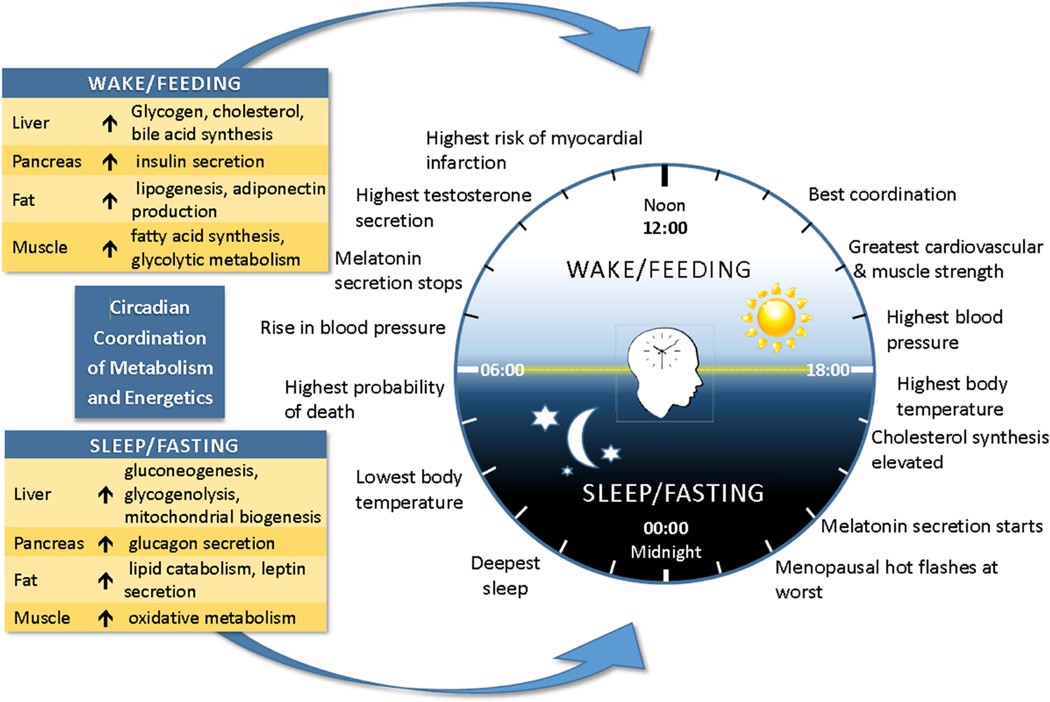 Many homeostatic, biological, and metabolic functions peak at different times of the day. In terms of energy metabolism, the morning/early afternoon seems to be the time when we will optimally metabolize what we eat. Source: J Acad Nutr Diet. 2015 Aug; 115(8): 1203?1212.
Many homeostatic, biological, and metabolic functions peak at different times of the day. In terms of energy metabolism, the morning/early afternoon seems to be the time when we will optimally metabolize what we eat. Source: J Acad Nutr Diet. 2015 Aug; 115(8): 1203?1212.
Sutton et al (Pennnington Biomedical Research Center) provide data for a more ?circadian-centric? dietary approach to TRF, providing efficacy in a group of prediabetic men and stimulating some interesting discussion on just when we should eat.
If you like this article, you?ll love my podcast, where I explore similar topics. Check it out on iTunes!
Study #1
?Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes?
Sutton et al (Pennnington Biomedical Research Center) provide data for a more ?circadian-centric? dietary approach to TRF, providing efficacy in a group of prediabetic men and stimulating some interesting discussion on just when we should eat.
What?d They Do?
To test whether 1) eTRF can improve cardiometabolic health and 2) IF (TRF) can have benefits independent of weight loss and food intake, authors performed a 5-week, randomized, crossover, isocaloric and eucaloric controlled feeding trial.
8 men completed both trials ? the TRF schedule and a control feeding schedule (not TRF) after a washout period. Isocaloric means both the control and TRF were matched for caloric intake. Eucaloric means that neither of these trials was designed to result in weight loss of the participants (in this they succeeded, neither group gained/lost any significant weight over 5 weeks).
TRF regimen: participants chose a habitual time between 6:30 and 8:30 am to start breakfast, and then lunch and dinner were timed accordingly (to end after a ~6 hour window, leading to ~18 hour fast period).
Control regimen: same breakfast time (self-chosen) but with a feeding window allowing for 12 hours total (participants ate lunch and dinner later, ending around 7:00 pm).
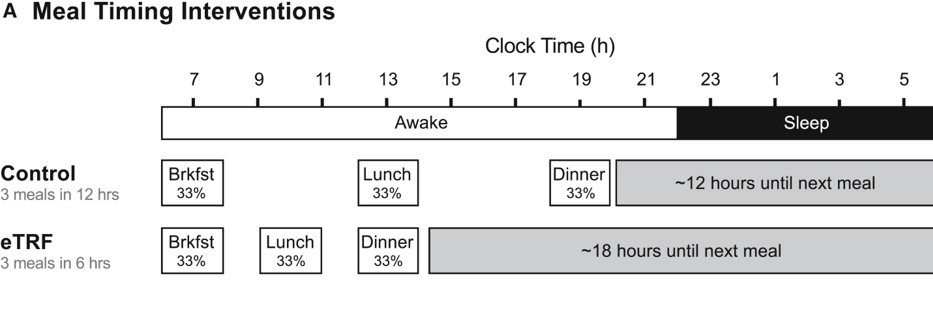 Sutton et al., 2018, Cell Metabolism 27, 1?10
Sutton et al., 2018, Cell Metabolism 27, 1?10
The quality of this study comes from how well-controlled the diet was. participants could only eat food given to them by the study staff, were fed enough food to maintain their weight (not gain/lose) and ate every singly meal while being monitored ? to ensure that they, you know, actually ate it. 100% of those in the eTRF arm and 98.9% in the control arm ate the provided meals.
Enjoying this story? Signup for my weekly email ?Physiology Friday? where I breakdown a study I found interesting during the week.
I want the newsletter!
What happened?
Glucose, Insulin, and Beta-cell function
Fasting glucose in the participants wasn?t any lower after the intervention, nor was glucose following an oral glucose tolerance test (OGTT, which measures how well the body metabolizes a load of glucose over a period of 3 hours).
However, the eTRF regimen did signigicantly affect insulin levels (the hormone responsible for regulating blood glucose). Fasting insulin and insulin levels during the OGTT (60 minutes and 90 minutes post-glucose load). This is important ? basically saying that the participants required less insulin to get the same amount of blood glucose indicating improved insulin sensitivity (important for prediabetics).
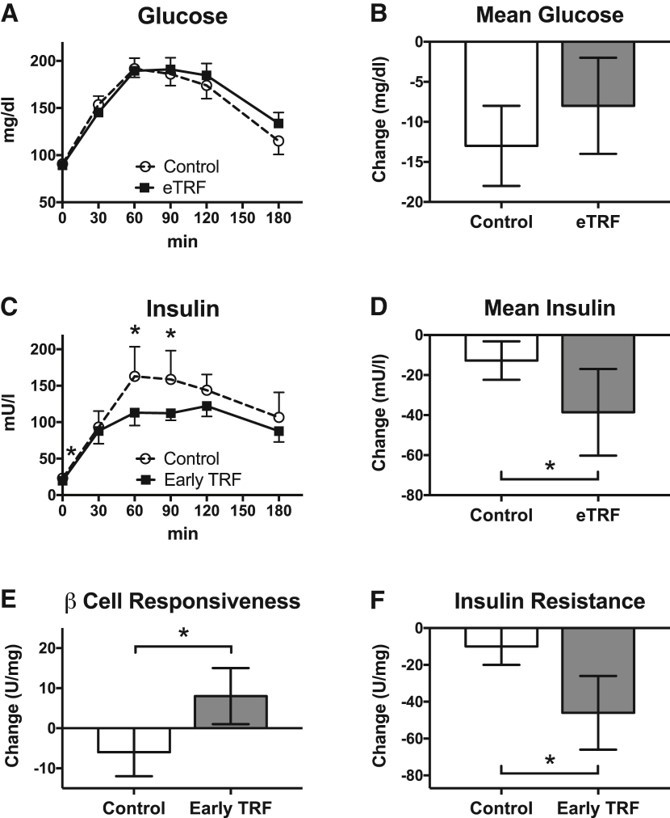
Early TRF can be used to effectively treat insulin resistance and improve pancreatic Beta-cell function
Note: Given that the fasting duration wasn?t matched before the OGTT or morning blood draws(i.e. 12 vs 18 hour fast in the respective groups), the authors suggest this may have influenced levels of glucose/insulin.
Cardiovascular Health Markers
Quite remarkably, 5 weeks of TRF lowered morning systolic and diastolic blood pressure ? by a pretty impressive amount. On average, SBP was 11 mmHg lower and DBP was 10 mmHg lower in the eTRF vs. control trial. The authors measured some others markers of arterial stiffness ? augmentation index (Aix) and pulse wave velocity (PWV) but found no significant results. Pretty typical for only a 5 week trial, as these measures sometimes take longer change in response to an intervention (like exercise).
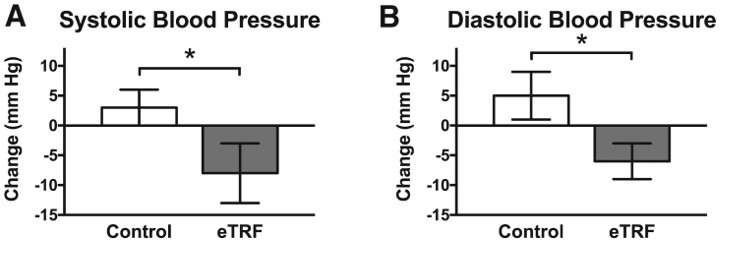 Systolic blood pressure and diastolic blood pressure both reduced in eTRF group vs. Control (11 mmHg and 10mmHg, for SBP/DBP, respectively).
Systolic blood pressure and diastolic blood pressure both reduced in eTRF group vs. Control (11 mmHg and 10mmHg, for SBP/DBP, respectively).
Another significant finding in this trial was that after eTRF, participants showed lower levels of a marker of oxidative stress ? 8-isoprostane. However, this was noted to be driven by an increase in the control arm ? so all that we know is that eTRF prevented oxidative stress from increasing over the trial period.
Weren?t They Hungry All of the Time!?
In short, it doesn?t seem like it. The researchers included a lot of subjective measures in their trial to assess ?diet? feasibility, tolerance, etc. Results indicated that, in the morning, no differences occurred in subjective measures of appetite between trials. Interestingly, theTRF group reported a reduced desire and capacity to eat in the evening, and hunger levels that were non-significantly different than the control arm. So ? stopping eating at 3 didn?t lead to any increase in hunger around dinner/bedtime.
The hunger hormones (ghrelin) and the ?satiated? hormone (leptin), notably, were not shown to be affected in the morning by either intervention. So, despite having a longer daily fasting duration, eTRF didn?t seem to increase hunger when food intake was matched throughout the day.
Interesting was the finding of lower desire/capacity to eat in the evening, which was probably both a behavioral and metabolic adaptation to the schedule (it took 12 days (range 3?35) to ?adjust? to the schedule)?after which the participants no longer felt like they needed to eat in the evening. This has important implications for compliance and feasibility for such interventions like these. If we will ever successfully implement these types of regimens into disease treatment, people are going to, first of all, have to do them.
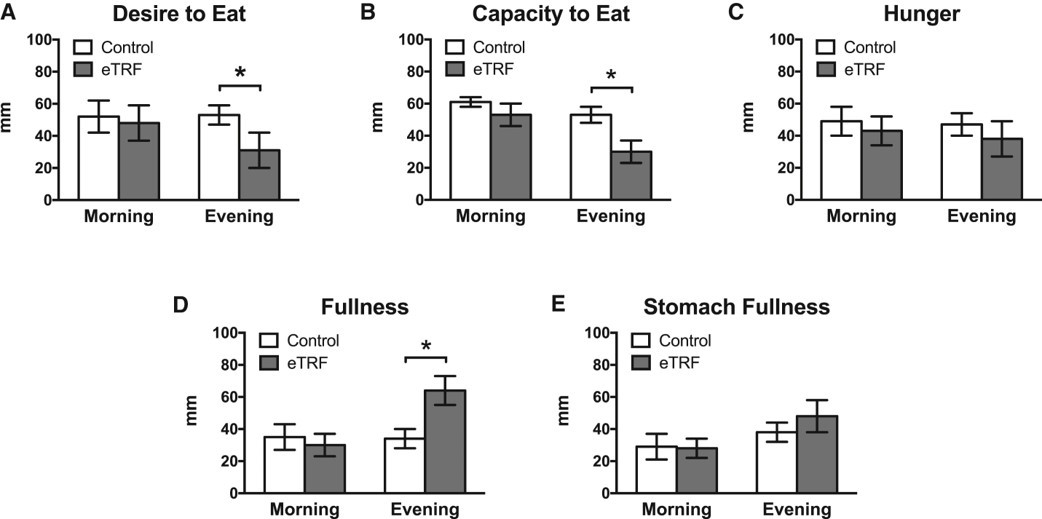 A lower subjective desire and capacity to eat in the evening was reported in the eTRF group. No differences in hunger were apparent between groups, but the eTRF group did report greater evening fullness.
A lower subjective desire and capacity to eat in the evening was reported in the eTRF group. No differences in hunger were apparent between groups, but the eTRF group did report greater evening fullness.
More interesting? The authors note that the main ?challenge? reported was not the 18 hour fasting window, or ending dinner around 3 pm. Most reported a challenge of eating within a 6 hour window during the day. Most thought that eating within a 7.8 hr period would be feasible for most people. Seems to be an inherent limitation to how much food someone can consume (or wishes to consume) within such a limited window of 6 hrs.
Study #2
?Early Time?Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans?
This one comes from researchers from the Pennington Biomedical Research Center (same as above) and the University of Alabama Birmingham (UAB).
Peterson et al. investigate early time restricted feeding (eTRF) in a cohort of ?healthy? adults and provide evidence that eTRF may benefit weight loss by mechanisms related to fat oxidation and appetite suppression, rather than increased energy expenditure, as suggested by some earlier studies in rodents.
What Did They Do?
In a randomized, crossover design, 11 participants were exposed to two different meal-timing regiments, each for a period of 4 days. The interventions involved 1) early time restricted feeding (eTRF) or 2) ?standard? feeding representative of a typical american eating pattern. Between each of the two diets, a 3.5?5 week washout period was implemented.
The diets were isocaloric and designed to maintain participants weight (i.e. energetic balance). They controlled for this by estimating participants resting energy expenditure and making dietary intake recommendations based upon matching energy intake to output.
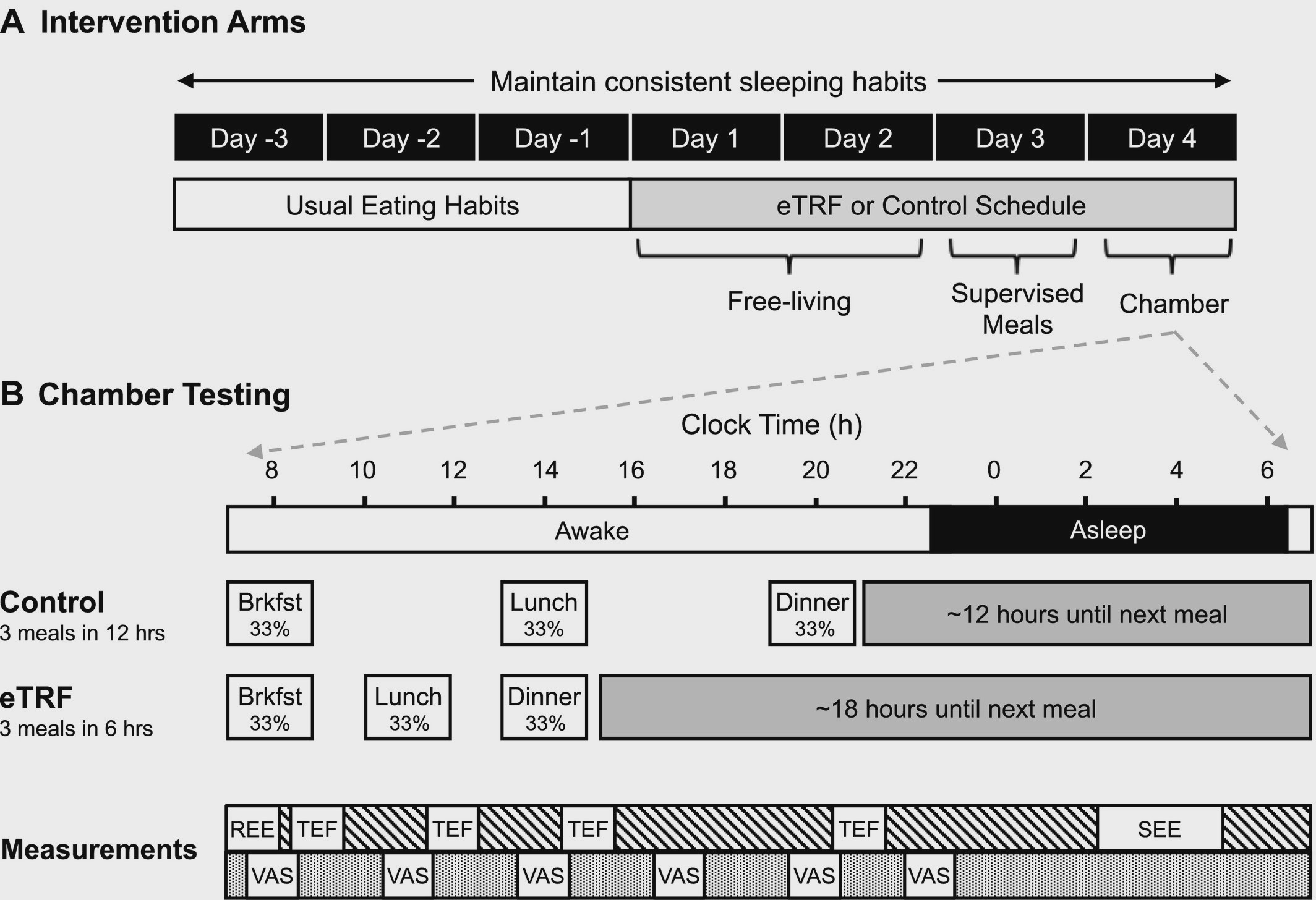 Study Design
Study Design
On days 1?2 of the diet, participants were ?free living? ? meaning they consumed their meals at home (not provided). They still ate at their prescribed meal times based on their group randomization. On day 3, participants were given supervised meals in the lab. On day 4, everyone was placed inside of a respiratory chamber, where they consumed 3 identical meals while having measures made of indirect calorimetry ? which essentially measures oxygen consumption and carbon dioxide generation in order to analyze metabolism, energy expenditure, and substrate (fat, carbohydrate, protein) utilization.
eTRF Diet
Designed to shift meal times to earlier in the day and compress the feeding window in to 6 hours. Meals were eaten at 8 am, 11 am, and 2 pm. This left for an ~18 hour fasting window.
Control Diet
Designed to emulate the typical pattern of eating for most people, stretching the feeding window to 12 hours. Meals were eaten at 8 am, 2 pm, and 8 pm. This left a more meager ~12 hour fasting window.
Study Measures
The major study outcome was 24 hour energy expenditure. In addition, substrate utilization, ?metabolic flexibility?, hunger hormones (ghrelin, leptin, glucagon-like peptide, peptide YY) and subjective ratings of hunger and energy were analyzed and compared among the two diets.
What Did They Find?
First and foremost ? overall 24 hour energy expenditure didn?t budge. While eTRF DID increase daytime energy expenditure by a mere 56 calories/12 hours, it led to a REDUCED energy expenditure throughout the night (-46 calories/12 hours) in comparison to the control diet ? things evened out. A majority of the increase in energy expenditure was theorized to come from the thermic effect of food (TEF) during the daytime feeding period. TEF is a measure of how much energy is takes to digest/metabolize a given meal. TEF is the reason you get hot after eating a meal (particularly one high in protein).
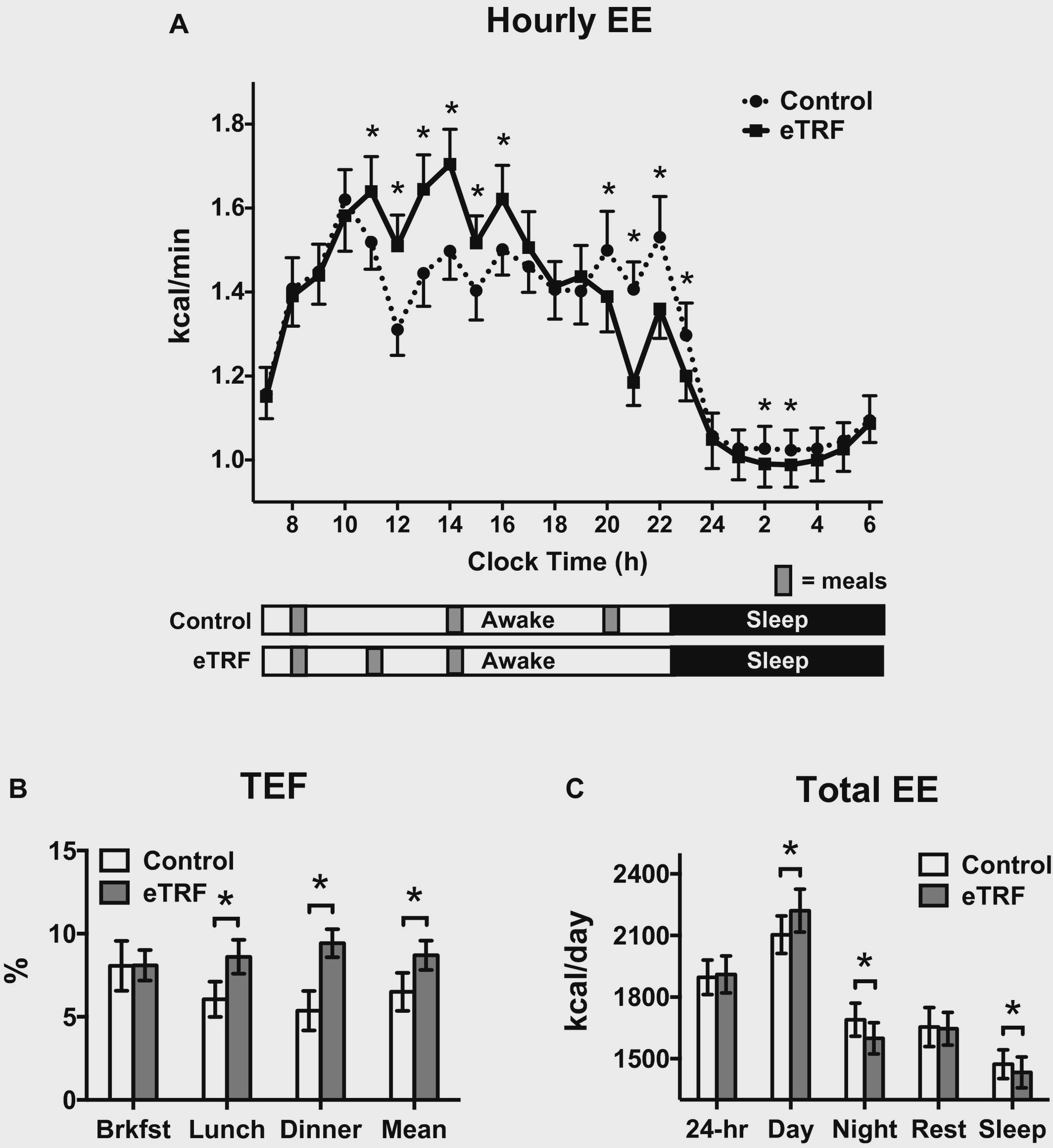 Hourly energy expenditure (EE), thermic effect of food at each meal (TEF), and total EE
Hourly energy expenditure (EE), thermic effect of food at each meal (TEF), and total EE
While energy expenditure wasn?t different, WHAT substrate contributed to energy expenditure did. The eTRF regimen led to a greater fat burning capacity oer 24 hours. This was measured using something called the non-protein respiratory quotient (npRQ) ? which measures the ratio of carbohydrate to fat oxidation from the expired gas analysis of indirect calorimetry described earlier. A lower RQ (around .7) indicates moer fat oxidation, while numbers closer to 1 indicate carbohydrate oxidation.
Fat oxidation increased, but so did something called metabolic flexibility. This term is used to describe someone?s ability to ?switch? between using fat and carbohydrate sources of energy depending on the situations. For instance, when you eat a high-carb meal, you want to be ?flexible? enough to release insulin and metabolize all those carbohydrates. Someone who is ?inflexible? (possibly insulin insensitive) may have a harder time adjusting to this demand. Flexibility also implies that once all that glucose runs out, you can switch back to utilizing fat for energy.
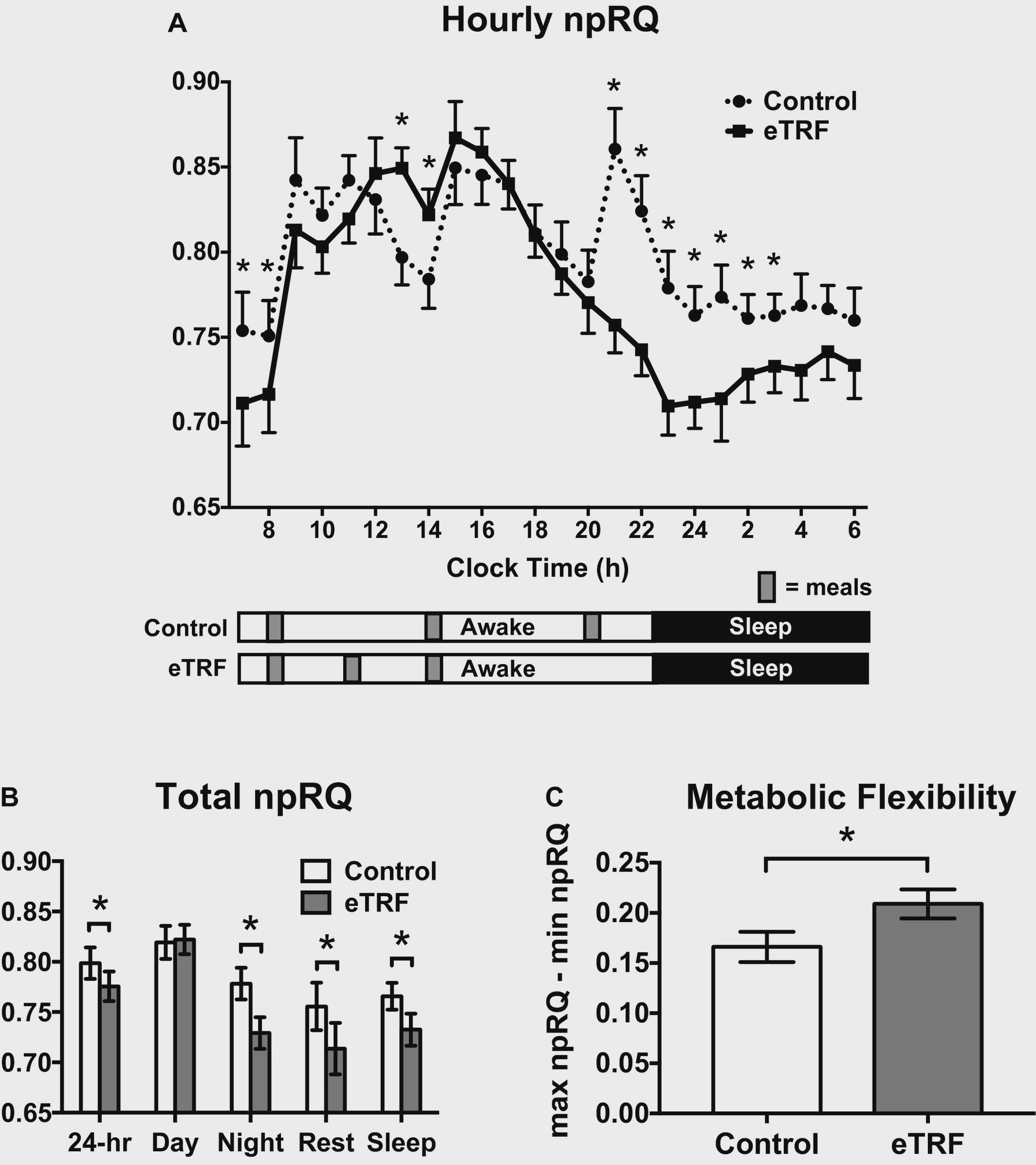 Non-protein respiratory quotient (npRQ) and metabolic flexibility
Non-protein respiratory quotient (npRQ) and metabolic flexibility
Anyhow, eTRF was shown to increase metabolic flexibility as well. In addition, eTRF increased protein oxidation compared to the control diet.
Increased fat burning makes sense ? eTRF participants were fasting for an average of 18 hours every day, and likely had run out of available glucose to use, and thus needed to rely on internal fat stores for energy.
The final relevant findings deal with subjective experience while on the eTRF regimen. Many are afraid or worried that eating a super late dinner or perhaps skipping dinner entirely will lead to the late-night munchies or a ravenous appetite. Results don?t support this conclusion. In fact, the eTRF group reported that their feelings of hunger decreased during the morning and mid-day, while feelings of fullness actually increased. This may have been due to hormonal influences. The ?hunger hormone?, ghrelin, was reduced in the morning in the eTRF regimen. In parallel, peptide YY, which signals satiety, increased in the mid-afternoon. Each of these most likely played a role in appetite modulation in response to eTRF.
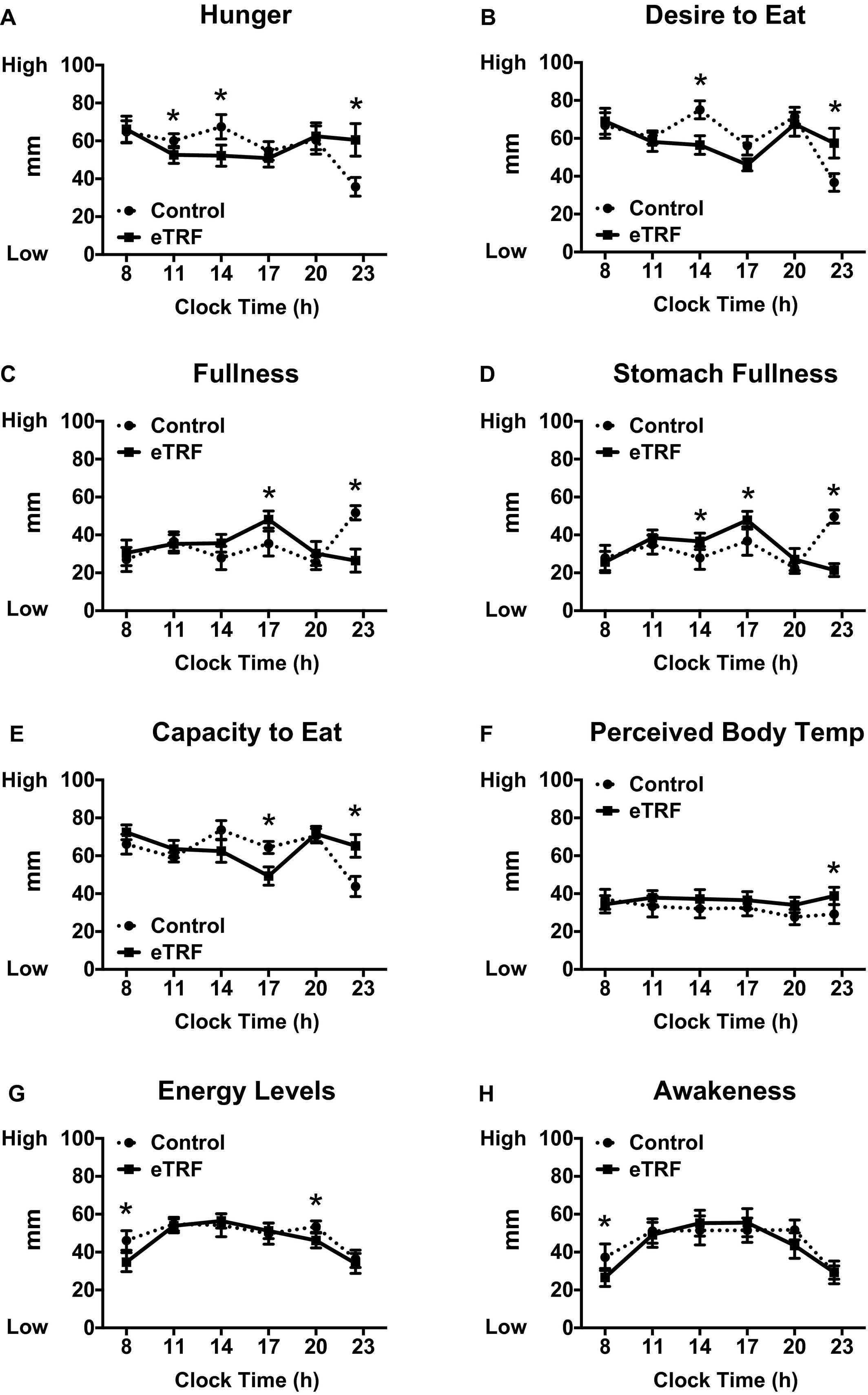 Subjective ratings of appetite and energy
Subjective ratings of appetite and energy
In addition to the initial study in pre-diabetic men, this controlled trial in ?healthy? adults lends further support in favor of eTRF. Even without reducing calories or inducing weight loss, substantial metabolic benefits were observed in just 4 days of early time restricted feeding.
In sum
An eating regimen involving early time restricting feeding (eTRF), when it is matched in food intake/calories to a ?control? diet, results in superior increases in insulin sensitivity and beta-cell function, lowers insulin levels, blood pressure, and reduces oxidative stress in prediabetic men. In addition, eTRF improves fat oxidation and metabolic flexibility in ?healthy? adults without disease. Importantly, these improvements occurred without any weight loss ? indicating that the benefits occur due to a mechanisms involving eating in alignment with circadian rhythms or perhaps appetite regulation. eTRF is a practice with increasing relevance and efficacy, especially in disease populations.

Sources
Sutton et al. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell metabolism (27) 1?10. June 5, 2018
Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation But Does Not Affect Energy Expenditure in Humans. Obesity (Silver Spring). 2019;27(8):1244?1254.
Patterson et al. Intermittent Fasting and Human Metabolic health. J Acad Nutr Diet. 115(8): 1203?1212. August 2015


