Did you know that the atomic model has been changed over a long period of time?When scientific knowledge develops, scientists learn more and their ideas about the atomic model change..This is a story of how the atomic model gets changed when new evidence comes along.Here is a timeline of some of the major ideas.
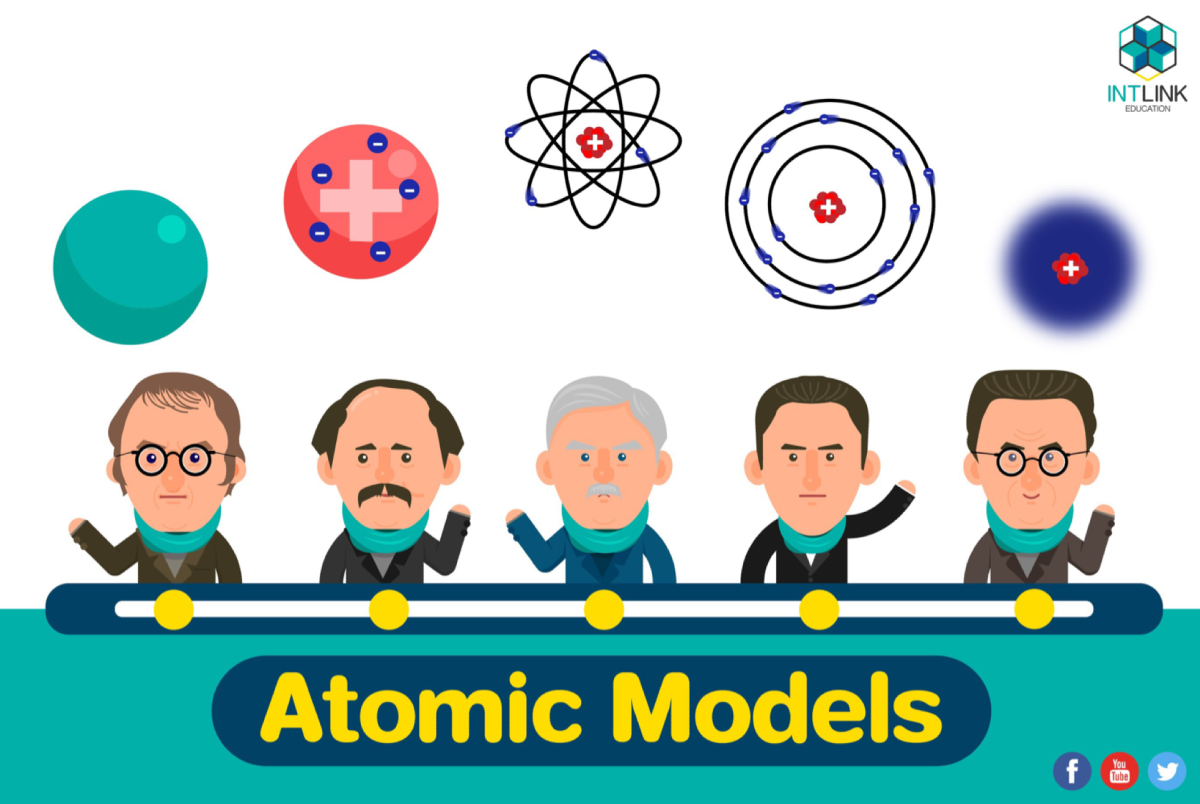
A timeline of atomic models
- Atomic model (1808)
- Plum-pudding model (1904)
- Nuclear model (1911)
- Planetary model (1913)
- Quantum mechanical model (1926-present)
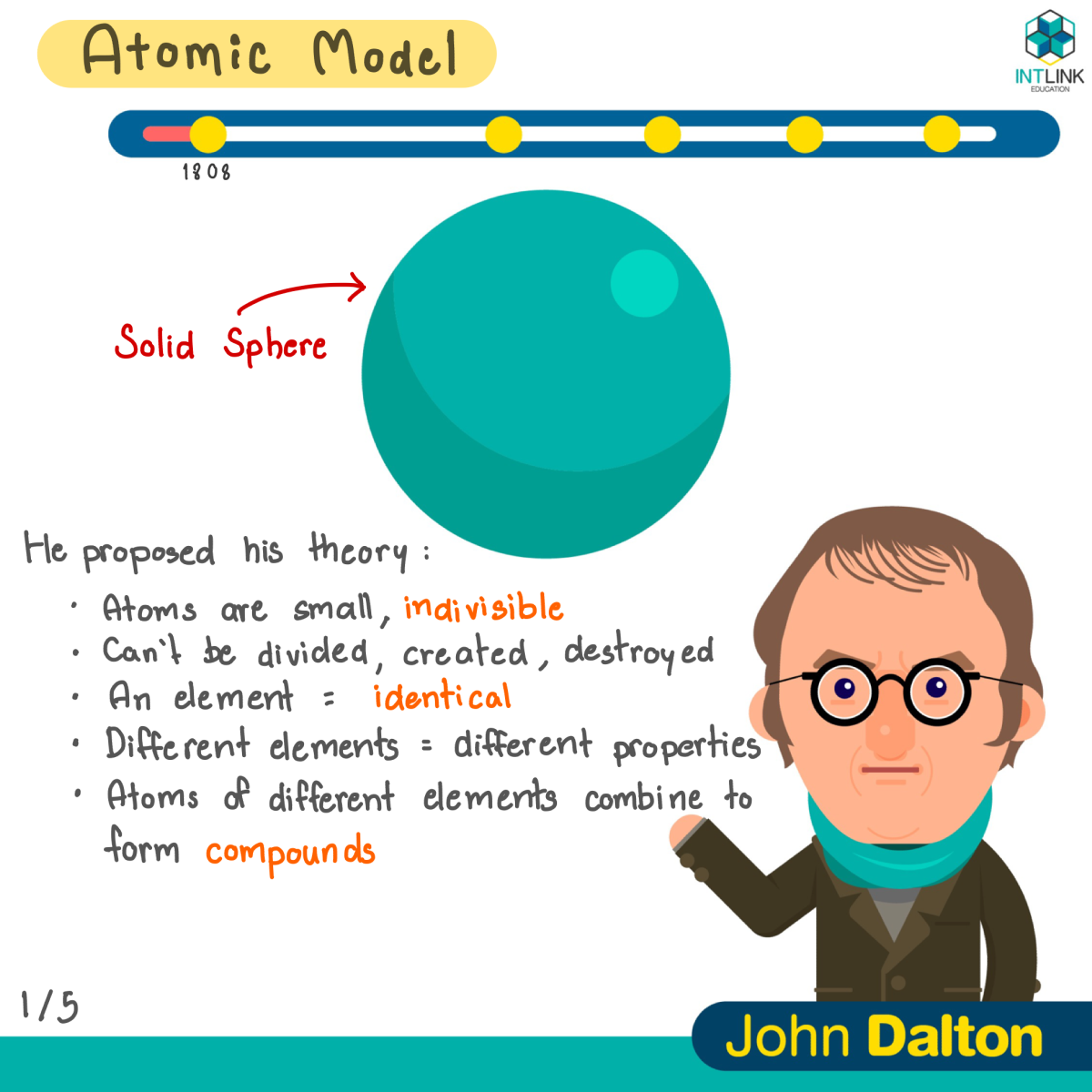
Atomic model: John Dalton
- Matter is made of small indivisible atoms.
- Atoms can?t be subdivided, created or destroyed. 2.1 Atoms of the same element have the same property. 2.2 Atoms of different elements have different properties.
3. Atoms of different elements can form compounds.
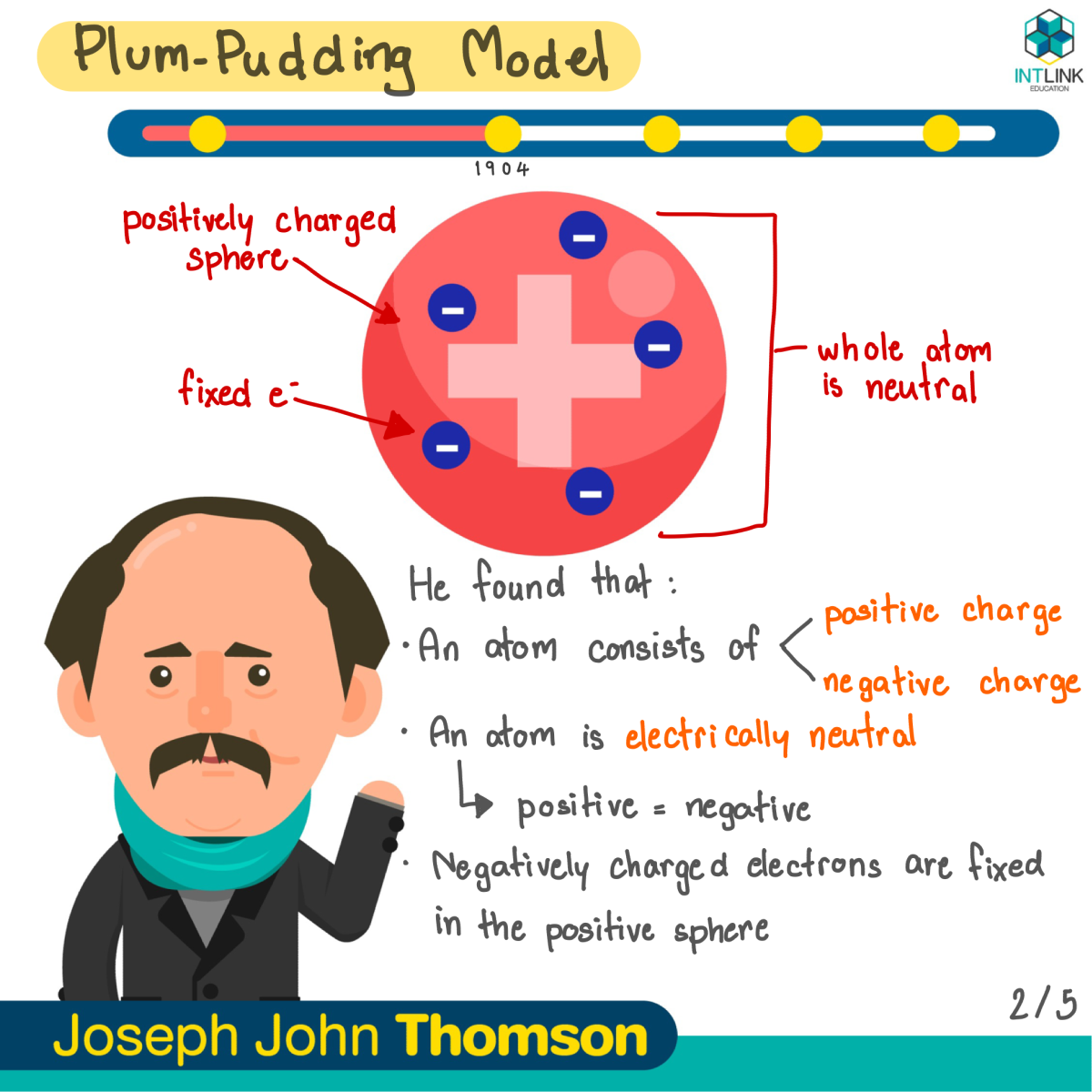
Plum-pudding model: J.J. Thomson
- An atom is electrically neutral. It has no charge.
- In an atom, both positive charges and negative charges are equal.
- An atom is made out of a sphere of positive charges with negatively charged electron embedded in it.
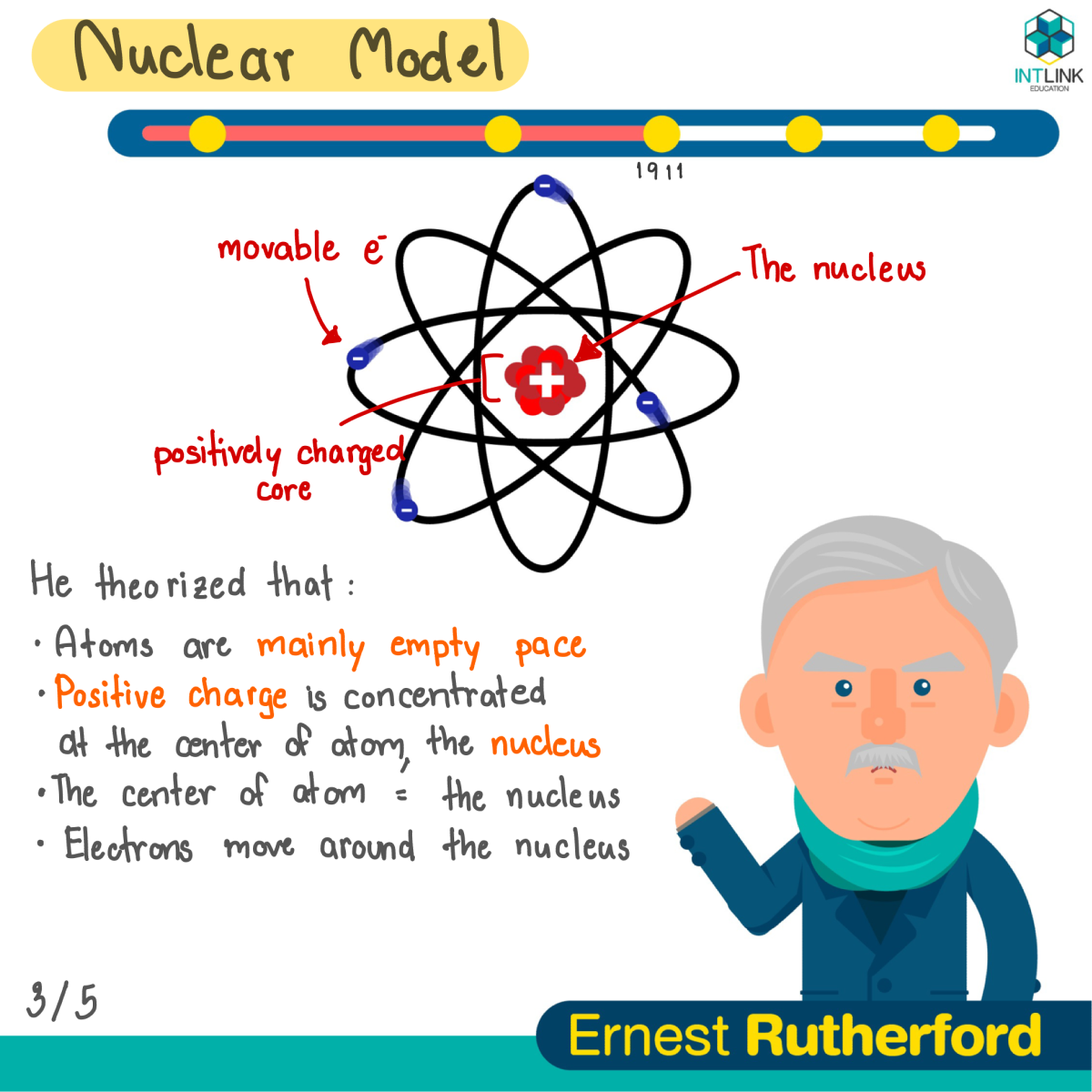
Nuclear model: Ernest Rutherford
- Atoms are mostly empty space.
- Most of the mass is concentrated in the center of atom. This tiny, dense, positively charged core called a nucleus.
- Electrons are located outside the nucleus.
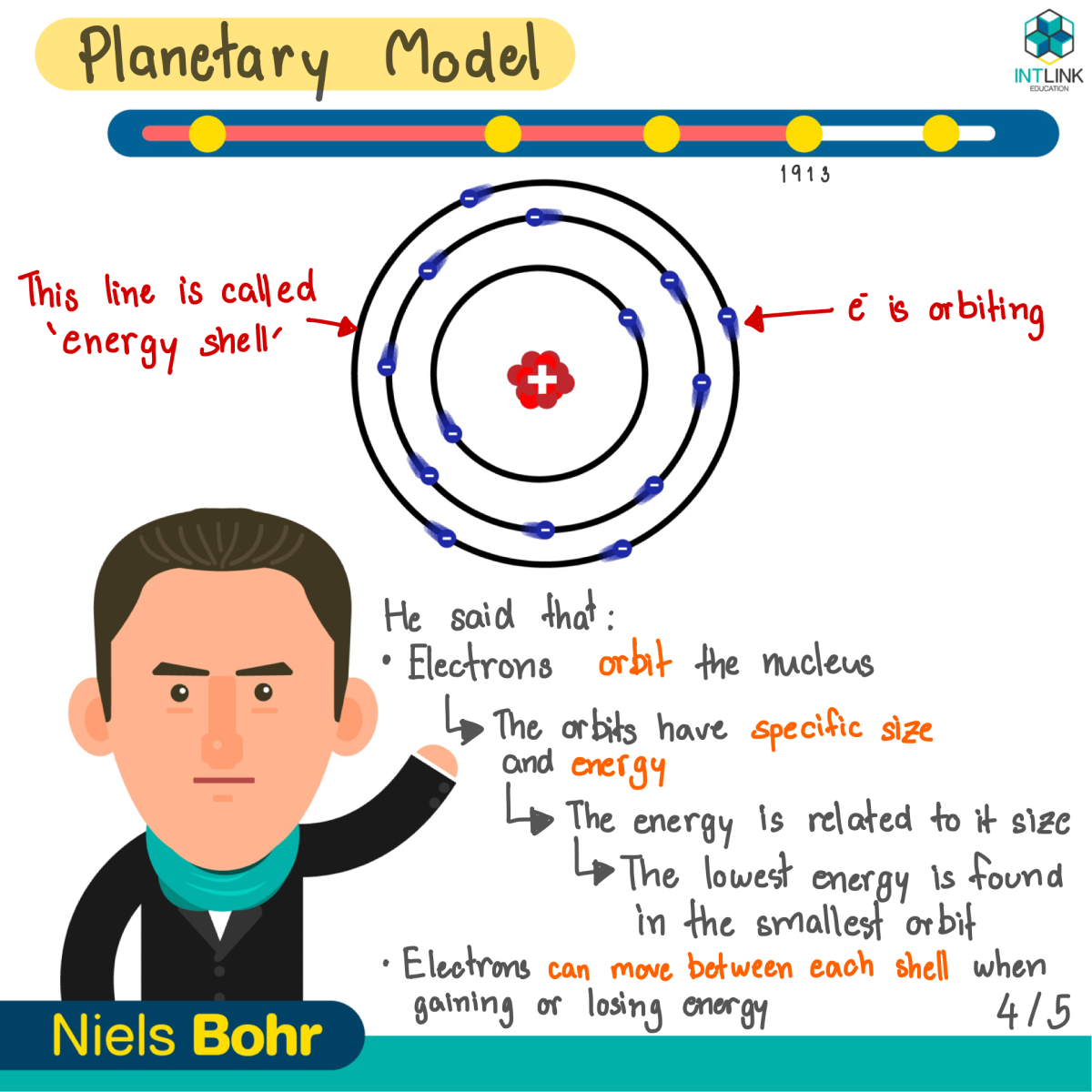
Planetary model: Niels Bohr
- Electrons orbit the nucleus in orbits that have specific size and energy.
- The energy of the orbit is related to its size. The lowest energy is found in the smallest orbit.
- Electrons reside in orbits. They move between each shell when gaining or losing energy.
- When gaining energy, electrons move to farther orbit from the nucleus. When losing energy, electrons move to closer orbit from the nucleus.
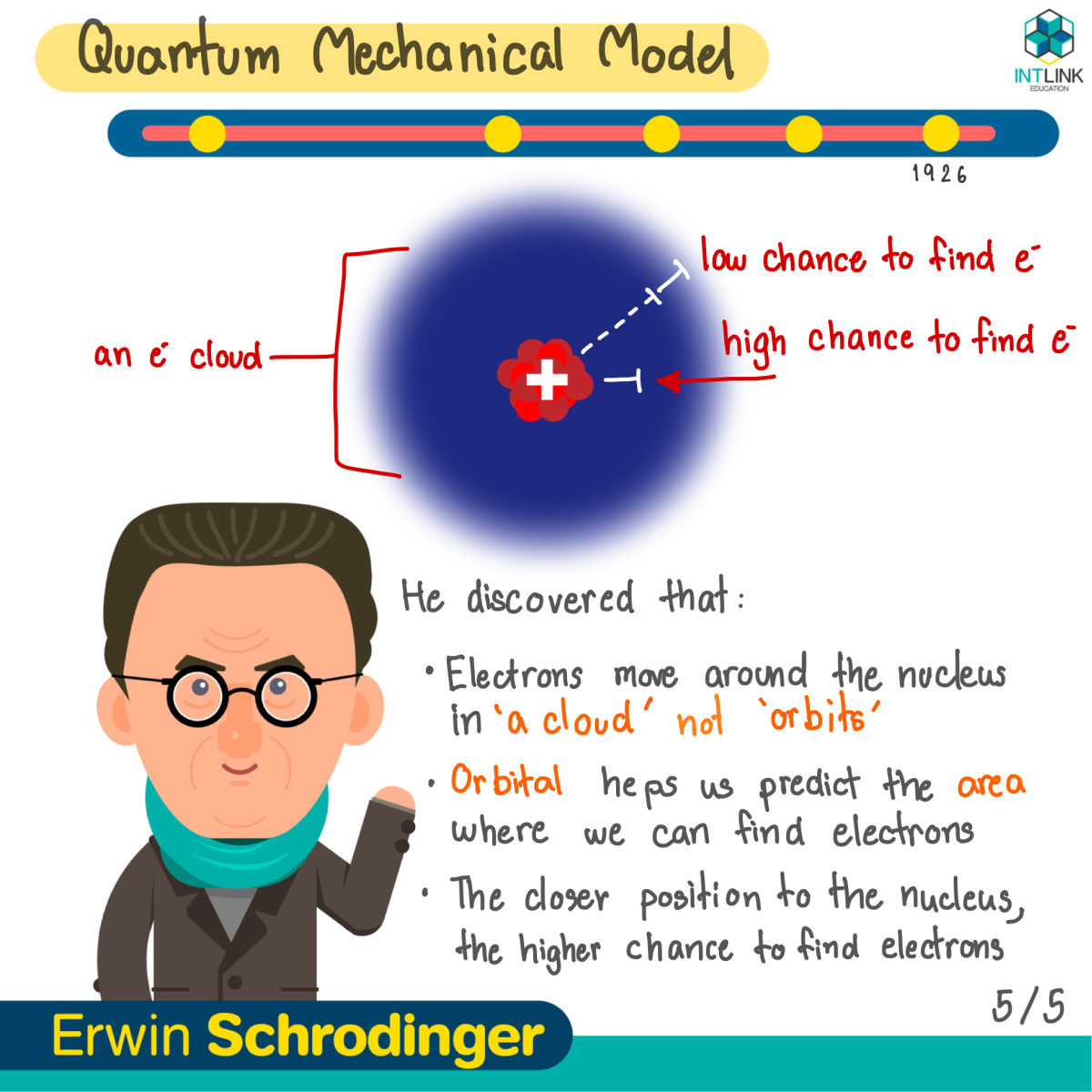
Quantum Mechanical Model: Erwin Schrdinger
- Electrons don?t move around the nucleus in orbits.
- Electrons exist in specific energy levels as a cloud.
- The electron cloud is the region of negative charges, which surrounds the nucleus.
- Orbital : The region with a high probability of containing electrons.
======================= Don?t forget to follow us for more updates on chemistry revision notes and tricks to improve yourself.
Facebook: https://www.facebook.com/IntLink.Education/ Twitter: https://twitter.com/Intlink3