/
/
8 Chemistry Trends Across The Periodic Table Explained
8 Chemistry Trends Across The Periodic Table Explained
1. Force of Attraction
- Definition: How much the outer electrons (-) are attracted to the nucleus (+)
- Increases going up periodic table because there is one fewer energy level of electrons that will separate the outer electrons from the nucleus
- Increases going right across periodic table because the nucleus gains protons and the atom has the same number of energy levels as you move across a period
2. Shielding Effect
- Definition: How much the outer electrons are repelled by inner electrons (negative repels negative), and also describes how much control the nucleus has on outer electrons
- Increases going down periodic table because there is an extra energy level of electrons that will shield the outer electrons from the nucleus
- Increases slightly going left on periodic table because the nucleus loses protons and the atom has the same number of energy levels as you move across a period
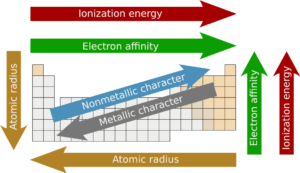
3. Atomic Radius
- Definition: A measure of a neutral atom?s size based on the radius of its volume as a sphere
- Increases going down because there are more energy levels of electrons and therefore more shielding
- Increases going left because as you move across a period right-to-left, an atom loses a positively charged proton and therefore its electrons become slightly less tight and compact
4. Ionic Radius
- Definition: Same as atomic radius, but for the size of a charged ion, not a neutral atom
- Increases for anions (-) because they gain an electron, which leads to more shielding
- Decreases for cations (+) because they lose an electron, which means less shielding
5. First Ionization Energy
- Definition: The amount of energy needed to remove an outer electron from an atom and make it into an ion
- Increases going right because there are more protons in the nucleus pulling in electrons, so it requires more energy to remove an outer electron
- Increases going up because there are fewer energy levels and less shielding, so the outer electrons are more tightly held by the nucleus
6. Metallic Character/Metallic Reactivity
- Definition: The tendency of an atom to lose an electron (a key characteristic of metals is they lose electrons to become cations)
- Increases going left because metals are on the left side of the periodic table and they have fewer protons in their nucleus that are holding in the atom?s electrons
- Increases going down because there is more shielding, so outer electrons are easier to lose
7. Non-Metal Reactivity/Electron Affinity
- Definition: The tendency of an atom to gain an electron (a key characteristic of non-metals is they gain electrons to become anions)
- Increases going right because non-metals are on the right side of the periodic table and they have more protons in their nucleus that attract outer electrons
- Increases going up because there are less electron energy levels and therefore a greater attraction to the nucleus for electrons
- ***Fluorine has greatest non-metal reactivity because noble gases are not included due to the fact that they have a full shell of valence electrons and cannot gain more or react
8. Electronegativity
- Definition: The tendency of an atom to attract a shared electron in a chemical bond
- Increases going right because there are more protons in the nucleus attracting outer electrons
- Increases going up because there are fewer energy levels of electrons shielding/repelling new outer electrons
- ***Fluorine has greatest electronegativity because noble gases are not included due to the fact that they have a full shell of valence electrons and cannot attract more