What if delaying diseases of aging, from cardiovascular disease to cancer to Parkinson?s disease, was as easy as eating whatever you wanted, but limiting your eating times to an 8-hour window every day or going on a prolonged fast every few days?
Learn more about intermittent fasting for health and fitness at lifefastingtracker.com. LifeOmic, a health software company headquartered in Indianapolis, Indiana and creator of the LifeOmic Precision Medicine Platform, has developed a mobile app that helps you harness intermittent fasting to kickstart your journey to healthier aging.
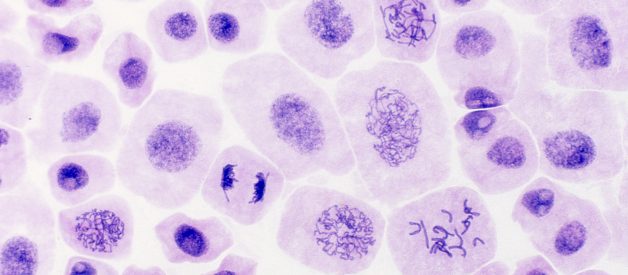
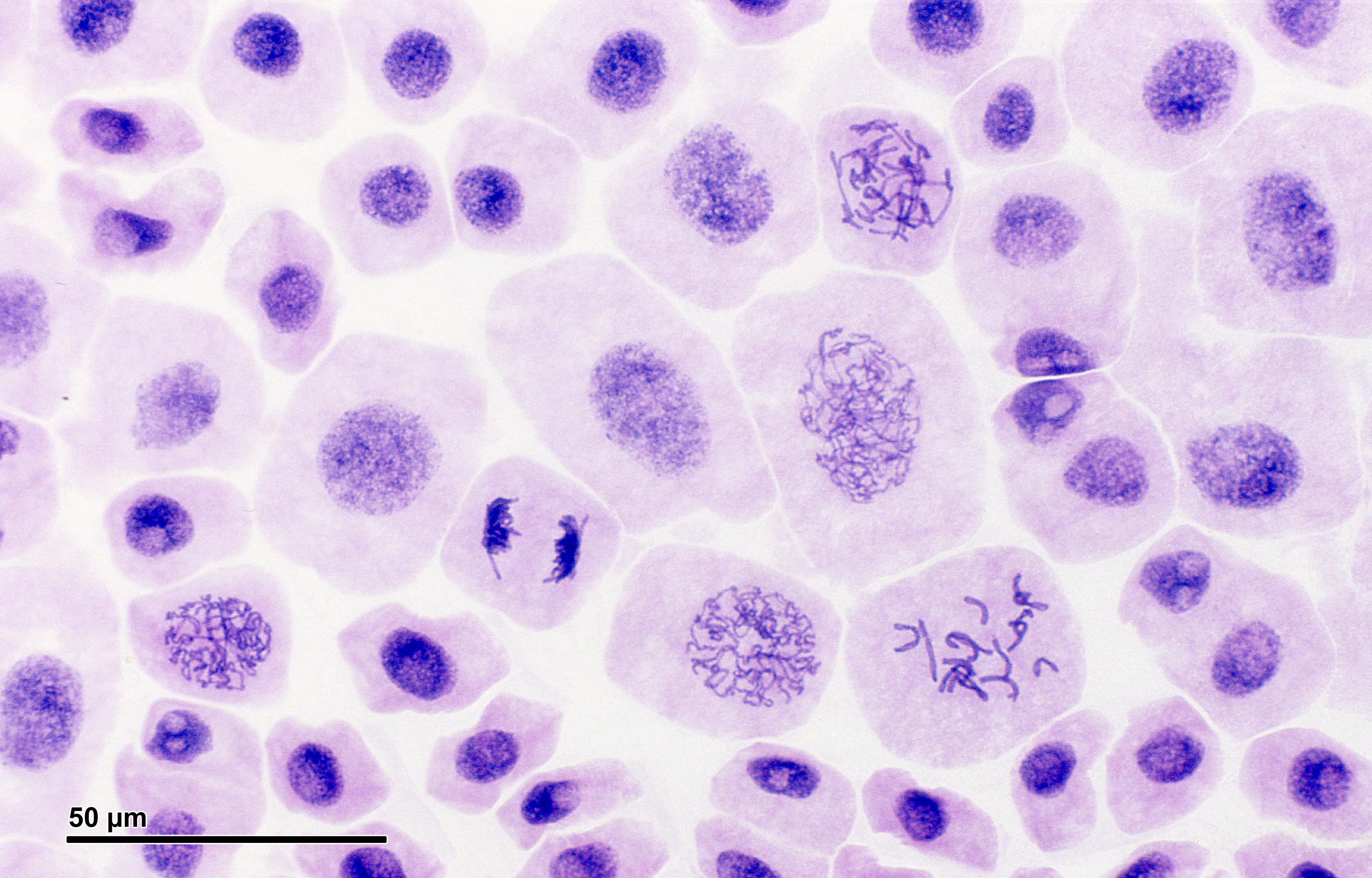 After a certain number of divisions, cells may become damaged and are forced into an early retirement state, called senescence, in which they pump out inflammatory signals that can promote aging. One of the metabolic signaling pathways that keeps these senescent cells going is the insulin signaling pathway initiated when you eat carbohydrates. Image: Mitosis, or cell division, in an onion. Credit: Doc. RNDr. Josef Reischig, CSc., Wiki.
After a certain number of divisions, cells may become damaged and are forced into an early retirement state, called senescence, in which they pump out inflammatory signals that can promote aging. One of the metabolic signaling pathways that keeps these senescent cells going is the insulin signaling pathway initiated when you eat carbohydrates. Image: Mitosis, or cell division, in an onion. Credit: Doc. RNDr. Josef Reischig, CSc., Wiki.
Ok, so it might not be that easy to avoid these infamous diseases, the risks of which increase as we age. Intermittent fasting clinical research has a long way to go. But researchers and clinicians are beginning to understand that these diseases are interconnected in more ways than we previously thought. In turns out that diseases we associated with aging are intimately connected to metabolism.
 Credit: Sightone, Flickr.com.
Credit: Sightone, Flickr.com.
Theories of aging point to a variety of factors that control how and when we age. We age as we accumulate errors or mutations in our DNA, and the protective ends of our chromosomes, called telomeres, shorten with every cell division. Thanks to fascinating research studies conducted by Dr. Elizabeth Blackburn, currently President of the Salk Institute for Biological Studies, and Dr. Elissa Epel, a professor of psychiatry at University of California, San Francisco, we know that telomere shortening is exacerbated by chronic stress, lack of sleep, lack of exercise and unhealthy diet. Cellular stress in the form of free radicals and reactive oxygen species also ages us.
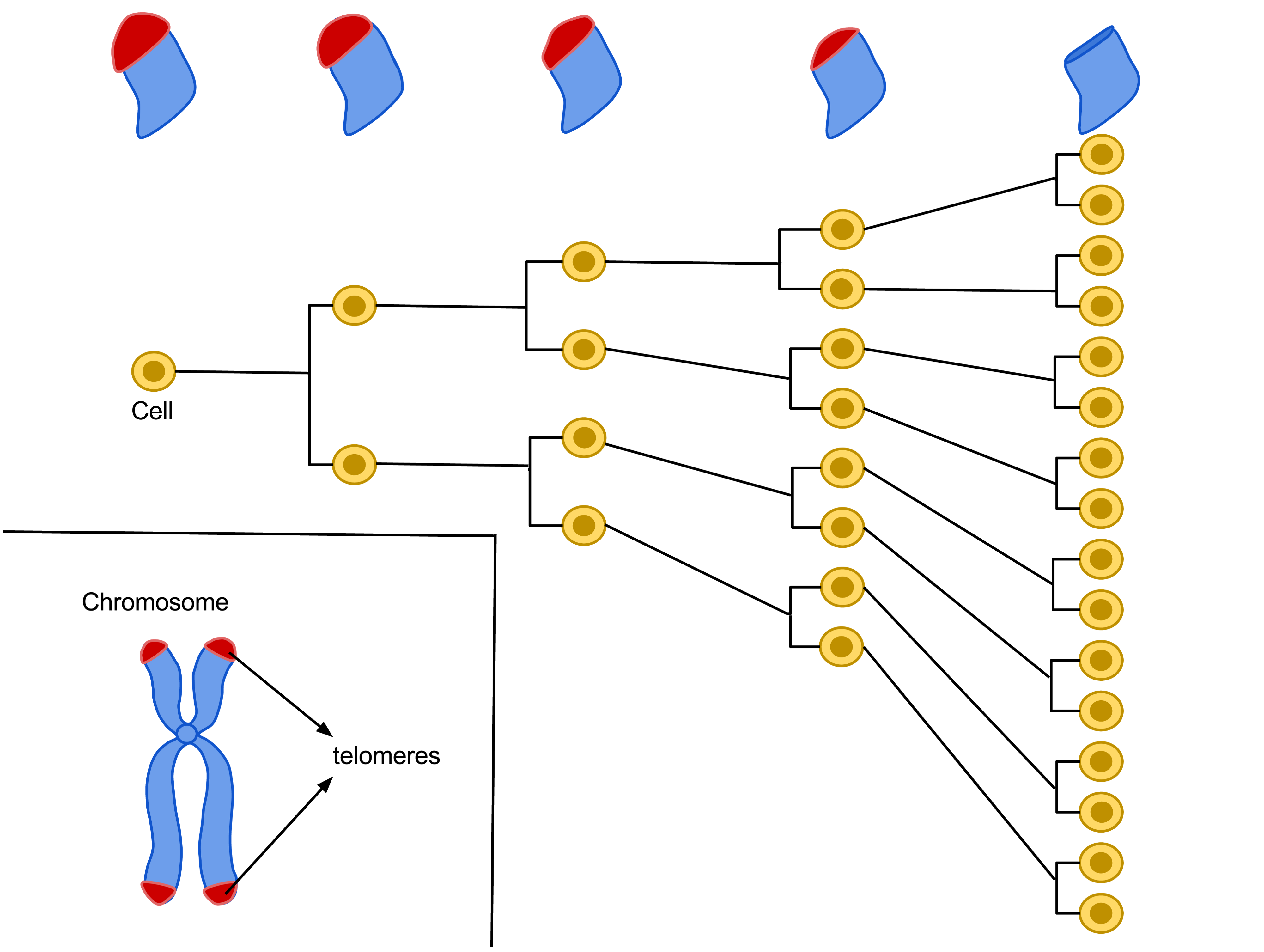 An illustration of the Hayflick limit, or the limit on the number of times a cell can divide due to shortening of its telomeres, which are liked the plastic tips on shoelaces that keep shoelaces, and DNA structures within our cells, from fraying. Illustration by Azmistowski17, Wikimedia.com.
An illustration of the Hayflick limit, or the limit on the number of times a cell can divide due to shortening of its telomeres, which are liked the plastic tips on shoelaces that keep shoelaces, and DNA structures within our cells, from fraying. Illustration by Azmistowski17, Wikimedia.com.
But one of the most recently discovered and popular theories of aging points to the role of cellular metabolism and insulin signaling in determining how functional our cells and tissues remain as we get older. Aging occurs not just to our bodies as a whole (wrinkles, gray hair, bone loss, etc.), but it also occurs within our individual cells, in the form of cellular senescence, DNA damage, and loss of metabolic flexibility.
One of the most potent signaling molecules for our cells is? insulin! In response to glucose (think: sugar), insulin prompts our cells to ?fire away? by activating various signaling pathways. Insulin stimulates cell growth and differentiation. But over time, an overabundance of ?grow and divide? signaling can encourage cells to remain active even when they are no longer healthy, for example when they have suffered internal damage or DNA damage. These unhealthy cells remain active but less than functional, pumping out inflammatory molecules that poison the healthy cells around them.
Take control of your insulin signaling? by fasting.
We know now that diseases of aging including cardiovascular disease, cancer, diabetes, and neurological diseases such as Parkinson?s and Alzheimer?s all have roots in cellular metabolism and senescence.
In recent years, scientific research has highlighted the role that metabolic interventions such as calorie restriction can play in extending lifespan. Senescent cells are far more intolerant to periods of starvation than our healthy dividing cells are. When deprived of glucose for 12 hours or more, our bodies, and cells, start burning fats for fuel, in process called ketosis. In metabolizing fats, ketosis produces ketone bodies that serve as fuel for our brain (our brain gets priority when our bodies think we are starving) and healthy cells in our bodies. But senescent cells, and cancer cells, are rather bad at being able to make the switch from using sugars to using fats as fuel. These cells have lost their metabolism flexibility, and we can use that to our advantage.
When the going gets tough in our bodies, senescent cells are marked for programmed death, giving way to newer, functional cells and revitalized tissues. Various studies have shown that calorie restriction extends the healthy portion of lifespan in worms, mice and monkeys, and perhaps even in humans, in part by lowering insulin and insulin-like growth factor concentrations, and lowering energy expenditure and oxidative stress (Katic & Kahn, 2005).
 In this 2009 photo are pictured rhesus monkeys, Canto, 27, and Owen, 29. The then 27-year-old monkey on the left was given a diet with fewer calories while the then 29-year-old monkey on the right was allowed to eat as much as it liked. The two were among the oldest surviving subjects in a long-term study of the links between diet and aging in Rhesus macaque monkeys. Photo credit: Jeff Miller, UW-Madison University Communications
In this 2009 photo are pictured rhesus monkeys, Canto, 27, and Owen, 29. The then 27-year-old monkey on the left was given a diet with fewer calories while the then 29-year-old monkey on the right was allowed to eat as much as it liked. The two were among the oldest surviving subjects in a long-term study of the links between diet and aging in Rhesus macaque monkeys. Photo credit: Jeff Miller, UW-Madison University Communications
In a serendipitous study of calorie restriction in humans, crew members enclosed in Biosphere 2 for two years experienced a limited but nutrient-rich diet, including lots of plant-based foods, that led to reduced blood pressure, cholesterol and tumor incidence, and improved insulin sensitivity.
 Biosphere 2, a serendipitous study of nutrient-rich caloric restriction in humans. Credit: Johndedios, Wiki.
Biosphere 2, a serendipitous study of nutrient-rich caloric restriction in humans. Credit: Johndedios, Wiki.
But calorie restriction doesn?t look, or feel, fun. Understandably, patient compliance to long-term calorie restriction regimens is very low ? it?s difficult to go most of your life eating only half of the calories your body craves. That?s where caloric restriction mimetics come in. How can we make our bodies think we are starving, and prompt our cells to clean up their act, without actually starving ourselves?
One answer may be intermittent fasting. Starving our bodies for just 12?16 hours begins to trick our cells into ?thinking? or acting like they might not get food for a long time. This triggers ketosis and processes that shut down senescent cells and their inflammatory secretions. Unlike most diet trends, fasting isn?t a fad. It?s a lifestyle that can boost lifespan and ?healthspan,? and is the subject of extensive scientific research in animal models and human clinical trials.
 Intermittent fasting is a hormetic stress. In other words, just like exercise, some amount of ?starving? your body for periods ranging from 12?24 hours at a time leads your body to mount a response that makes your cells become more efficient. Damaged cells that contribute to aging and disease-related inflammation are killed off. Intermittent fasting simply limits the time that your body is in a fed state, not overall amount or type of food.
Intermittent fasting is a hormetic stress. In other words, just like exercise, some amount of ?starving? your body for periods ranging from 12?24 hours at a time leads your body to mount a response that makes your cells become more efficient. Damaged cells that contribute to aging and disease-related inflammation are killed off. Intermittent fasting simply limits the time that your body is in a fed state, not overall amount or type of food.
Fasting has ancient roots in human history, but researchers only recently uncovered the molecular mechanisms that allow intermittent fasting to reduce inflammatory responses in our body, optimize energy metabolism, protect our healthy cells from damage and clear away damaged cells to make way for new ones. It all goes back to glucose/insulin signaling. Prolonged fasting can even activate stem cells and prompt a rejuvenation of our immune systems. Intermittent fasting or time-restricted feeding, which involves going without calories for at least 12 hours a day, can result in weight loss and lower risk of diseases of aging including heart disease, diabetes, neurodegenerative disorders, and even cancer. Intermittent fasting regimens might also influence our circadian biology and the healthy microbes that live in our guts in positive ways.
 LifeOmic LIFE app. Follow us on Twitter @LifeOmicIF.
LifeOmic LIFE app. Follow us on Twitter @LifeOmicIF.
At LifeOmic, we are passionate about improving human health and healthspan through big data and precision medicine. We have created a mobile app, the LifeOmic Intermittent Fasting Experience (LIFE) app, that harnesses the power of our precision medicine platform to help you make intermittent fasting a part of your routine and securely track your health outcomes. Our app can also help researchers and clinicians rigorously study the impact of health interventions such as fasting on markers of healthspan, including genetic and gene expression markers. (Interested? Contact us!)
The LIFE app makes it easy to set and achieve your objectives, within a social community of fellow fasters. We are the only intermittent fasting app that lets you connect view and interact around your circle?s fasting experiences in real time.
LifeOmic has deep scientific roots. The LIFE app includes information and intuitive feedback about when and how you?ll enter ketosis, and a learning library that allows you to understand and keep up with the rapidly evolving science behind intermittent fasting. Delve into the cellular mechanisms of intermittent fasting by means of easy to understand videos that explain how it works. You?ll enjoy and feel empowered by a stream of scientific information related to health and longevity that is updated regularly. LIFE makes it easy to integrate fasting into your lifestyle, track your progress, and share your experiences with people you care about.
Sign up to be a LIFE beta-tester and see for yourself how intermittent fasting can positively impact your metabolic and genetic health.