/
/
4 Steps to Naming Compounds in Chemistry Nomenclature
4 Steps to Naming Compounds in Chemistry Nomenclature
1. Is it an Ionic Compound or a Molecular Compound?
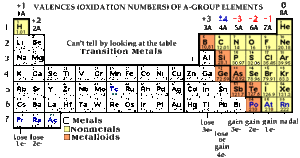
- It is Ionic if it has a metal (an element on the left or middle of the periodic table)
- It is Molecular if it has two nonmetals (elements on the right side of the periodic table above the staircase, plus hydrogen)
2. Add an ?ide? to the end of the second compound?s name
- For both molecular and ionic compounds, change the name of the second compound so it ends in ?ide?
- ex: fluorine = fluoride, hydrogen = hydride
3. See if you need roman numerals
- Check if an Ionic Compound has a transition metal that becomes a multivalent ion (AKA if the element is in the middle of the periodic table and you don?t automatically know its charge)
- For Ionic Compounds with transition metals, you need to insert a roman numeral after the name of the metal to indicate the transition metal?s charge
- ex: FeCl = Iron(I) Chloride and FeCl2 = Iron(II) Chloride
- Think about what the charge of the metal needs to be to balance out the nonmetal?s charge
4. See if you need prefixes
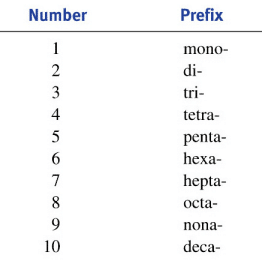
- Since there are no ionic charges to balance out in molecular compounds, you need to use prefixes (see chart)
- ex: N2O5 = dinitrogen pentoxide
- For the first element, you don?t need to say ?mono?
- ex: CO = carbon monoxide (not monocarbon monoxide)




